Which of the following ionsis paramagnetic?
[Ni(H2O)6]2+
[Fe(CN)6]4−
[Ni(CO)4]
[Ni(CN)4]2−
In [Ni(CO)4]
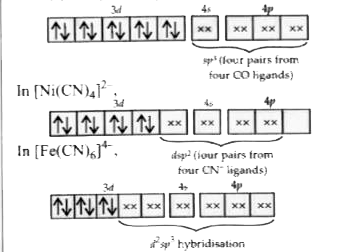
Strong field ligands like CO,CN− generally result in inner orbital complexes.
In [Ni(H2O6]2+,H2O is a weak field ligand, pairing of electrons will not take place. Hence [Ni(H2O)6]2+ is paramagnetic in nature.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - MTG-WBJEECHAPTER - COORDINATION COMPOUNDSEXERCISE - WBJEE/WORKOUT (Category 2: Single Option Correct Type)
13 Videos
- COORDINATION COMPOUNDSBOOK - MTG-WBJEECHAPTER - COORDINATION COMPOUNDSEXERCISE - WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)
7 Videos
- CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSBOOK - MTG-WBJEECHAPTER - CHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDSEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)
1 Video
- ENVIRONMENTAL CHEMISTRY BOOK - MTG-WBJEECHAPTER - ENVIRONMENTAL CHEMISTRY EXERCISE - WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )
2 Videos
Similar Questions
A complex compound of Co3+ with molecular formula CoClx.yNH3 gives a total of 3 ions on dissovling it in water.To satisfy both primary and secondary valencies in this complex, the number of Cl− ions required is
View SolutionIn which of the following cases, the complex ion formed will migrate towards the anode under the electric field?
View Solutionwhite phosphorus P4 has the following characteristics.
View SolutionAmong the followings , the one which is not a " greenhouse gas " , is
View SolutionWhich of the following show paramagnetism ? .
View SolutionWhich of the following comnpounds is paramagnetic?
View SolutionWhich of the following are paramagnetic?
View SolutionWhich of the following paramagnetic in nature
View SolutionWhich of the following complexes is(are) paramagnetic ?
View SolutionWhich of the following molecule is not paramagnetic :
View SolutionWhich of the following is highest paramagnetic
View SolutionWhich of the following are paramagnetic ?
View SolutionWhich is not paramagnetic of the following?
View SolutionWhich one of the following is not paramagnetic-
View SolutionWhich of the following is/are paramagnetic ?
View Solution

