When an ideal undergoes unrestrained expansion, no cooling occurs because the molecules
are above the inversion temperature
exert no attraction force on each
do work equal to loss in kinetic energy
collide without loss of energy.
When an ideal gas undergoes expansion, no work is done in overcoming attractive forces. Hence internal energy does not fall and hence temperature also does not fall
Topper's Solved these Questions
- STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)BOOK - MTG-WBJEECHAPTER - STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)EXERCISE - WBJEE/WORKOUT (Category 2: Single Option Correct Type)
15 Videos
- STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)BOOK - MTG-WBJEECHAPTER - STATES OF MATTER (SOLIDS, LIQUIDS AND GASES)EXERCISE - WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)
9 Videos
- REDOX EQUILIBRIABOOK - MTG-WBJEECHAPTER - REDOX EQUILIBRIAEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS
5 Videos
- SURFACE CHEMISTRYBOOK - MTG-WBJEECHAPTER - SURFACE CHEMISTRYEXERCISE - WB JEE PREVIOUS YEARS QUESTIONS
3 Videos
Similar Questions
Consider the following statements. If the van der Waals' parameters of two gases are given as a/dm6bar mol−2b/dm3−mol−1
GasA 6.5 0.056
GasB 18.0 0.011
then
1. critical volume A> critical volume of B
2. critical pressure of A> critical presure of B
3. critical temperature of A> critical temperature of B
Which of the above statements is/are correct?
View SolutionFor the orthorhombic crystal system
View SolutionThe two half cell reactions of an electrochemical cell is given as
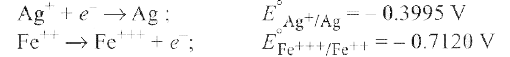
the value of cell EMF will be
View SolutionThe amount of electrolytes required to coagulate a give amount of Agl colloidal solution (-ve charge) will be in the order .
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionIf an ideal gas is allowed to undergo free expansion it does not cool. But a real gas cools during free expansion. Why?
View SolutionWhen an iden gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View Solutionजब एक आदर्श गैस में अनियंत्रित प्रसार (unrestrained expanision ) होता है , तो शीतलता (cooling) उत्पन्न नहीं होती , क्योंकि अणु
View SolutionWhen a gas undergoes adiabatic expansion, it gets cooles. This is because
View SolutionWhen an ideal gas undergoes unrestrained expansion, on cooling occurs because the molecules
View SolutionWhen an ideal gas expands in vacuum, no cooling is observed because the molecules
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen an ideal gas undergoes undestrained expansion, no cooling occurs because the molecules
View Solution(A) : Ideal gasses do not undergo cooling during expansion (R) : Molecules of ideal gas do not attract mutually
View Solution

