The compoiund (A0 is xylene. On sulphonation, it gives only one product (B) which on alkaine fusion followed by acidification yields (C), a phenol. On treatment with chlorform and alkali, (C) gives C9H1O2(D). Idenifuy the various organic compounds (A) to (D).
(A) is xylene, it can be at o,m, and p. But it gives only one substitude product, so (A) is p− xylene. (OMP=231).

Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Concept Application Type
Explore 2 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Concept Application
Explore 4 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Subjective Type
Explore 4 Videos
- APPENDIX INORGANIC VOLUME 2Book:CENGAGE CHEMISTRYChapter:APPENDIX INORGANIC VOLUME 2Exercise:Short Answer Type
Explore 179 Videos
- BIOMOLECULESBook:CENGAGE CHEMISTRYChapter:BIOMOLECULESExercise:Exercises Archives (Analytical And Descriptive)
Explore 8 Videos
Similar Questions
Two isomeric mononitro derivaters (B) and (C) are obtained by the nirtration of an organic compound (A),C7H8O. Treatment of (A0 with acetyl chloride products (D) which on reaction with CrO2Cl2 gives (E) whose oxidation with neutral KMnO4 followed by acidification gives (F). Compound (F) on heating gives pheonl. (A) on treatment with alkaline C6H5SO2Cl produces. (A) on treatment with alkaline C6H5SO2Cl produces (G) which on oxidiation with KMnO4 gives (H). Hydrolysis of (H) also gives (F). Give teh structures of (A) to (H).
View SolutionCompound C8H9Cl(A) on treatment with KICN followedd by hydrolysis gives C9H10O2(B). Ammonium salt of (B) on dry disillation yiels (C), wyhich reacts with alkene solution of bromnine to give C8H141N(D). Another compound C8H10O(E) is obtained by the actions by the action of nitrous acid of (D) or by the action of aqueosu potacsh on (A),(E) on oxidation gives the inner anthydide C8H6O4(F) which gaves the inner anthdride C8H4O3(G) on heating Identify the various compounds (A) to (G).
View Solution(a) Two moles of organic compounds 'A' on treatment with a strong base gives two compounds 'B' and 'C'. Compound 'B' on dehydrogention with Cu gives 'A' while acidification of 'C' yields carboxylic and 'D' with molecular formula of CH2O2. Identify the compound A, B, C, and D and write all chemical reactions involved.
(b) (i) o-nitrophenol has lower b.p. than p-nitrophenol. Explain.
(ii) Write IUPAC name of the following :
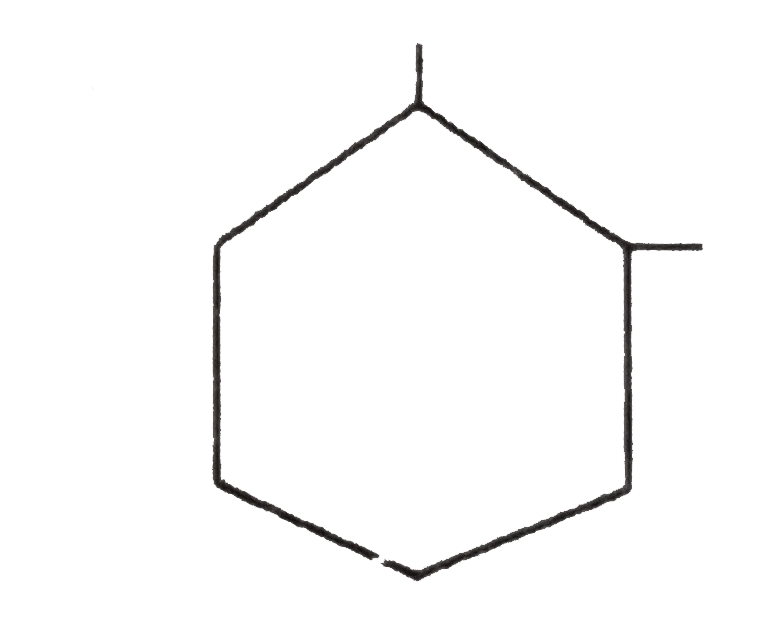
View SolutionTwo moles of organic compound 'A' on treatment with a strong base gives two compounds B and C. Compound 'B' on dehydrogenation with Cu gives 'A' while acidification of 'C yields carboxylic acid 'D' having molecular formula of CH2O2. Identify the compounds A, B, C and D.
View SolutionTwo moles of organic compound 'A' on treatment with a strong base gives two compound 'B' and 'C'. Compound 'B' on dehydrogenation with Cu gives 'A' while acidification of 'C' yields carboxylic acid 'D' with molecular formula of CH2O2 . Identify the compounds A, B, C and D and write all chemical reactions involved.
View SolutionA compound (A) C5H9Br on treatment with Mg in dry ether gives a compound (B) which on reaction with acetaldehyde gives an alcohol (C), on mild acidification prolonged treatment of alcohol with equivalent amount of HBr gives a compound (D) which on reaction with alcoholic KOH gives compound (E). compound (E) on ozonolysis followed by treatment with H2∅Zn gives (F). the compound (F) on treatment with base gives 1 acetyl cyclopentene (G). identify (A) to (G).
View SolutionAn organic compound (A) (C8H14O) forms an oxime and gives a positive haloform reaction. On ozonolysis, it gives acetone and a compound (B) (C5H8O2) . (B) forms a dioxime and on subjecting to oxidation reaction gives an acid (C ) (C4H6O4). On treatment with excess of ammonia and strong heating, (C ) gives a neutral compound (D) (C4H5O2N). (D) on distillation with zinc dust forms pyrrloe. Suggest the possible structures of (A), (B), (C ), and (D). Explain the chemical reactions involved.
View SolutionAn organci compound C6H4O2NCl(A) reacts with a,la,oo tp gove C6H5O3N(B). On reduction with tin and HCl,(B) gives C6H7ON(C) whereas on milder reduction it gives (D)C6H7O2N, which rearranges in the presence of a mineral acid to give a new compound (E). Identify the various compounds from (A), to (E).
View SolutionBromobenzene on treatment with fuming H2SO4 gives a compound which one treatment with Cl2/AlCl3 gives (A), (A) on distillation with dil. H2SO4 gives (B). (B) on treatment with an eq. of Mg in dry ether gives (C ). (C ) on treatment with CH3CHO followed by hydrolysis gives (D). (D) on treatment with NaOl gives (E). Identify the structures of (A) to (E).
View SolutionAn organic compound (A) C8H4O3 , in dry bebzene in the presence of anhydrous AlCl3 gives compound (B ). The compound (B) on treatment with PCl5 followed by reaction with H2/Pd(BaSo4) gives compound (C ) which on reaction with hydrazine gives a cyclide compound (D) (C14H10N2). Identify (A), (B) (C ), and (D) Explain the formation of (D ) from (C ).
View Solution

