β− Elimination or anti-eliminination reaction is carried out with base (Bo−) as shown below:
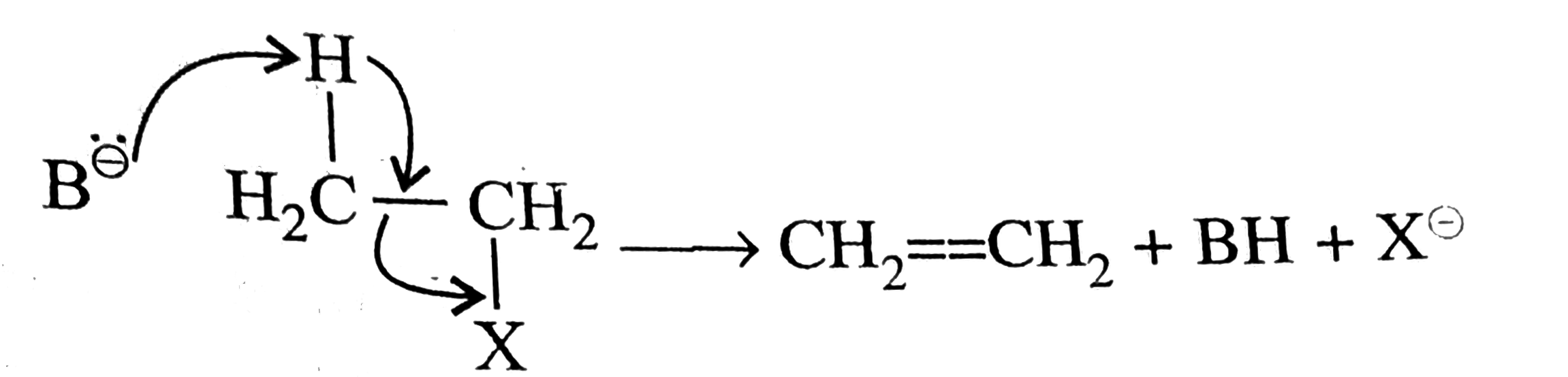
The following bases are used.
I. o−OH , II. ROo−
II. ROo−
III. RCOOo− , IV. o−CN
V. NOo−3
The decreasing order of reactivity for the above elimination is:
(II)>(I)>(IV)>(III)>(V)
(V)>(III)>(IV)>(I)>(II)
(II)>(I)>(III)>(IV)>(V)
(I)>(II)>(III)>(IV)>(V)
The correct Answer is:a
The regent should be a strong Bronsted base.
Acidic order: HNO3>RCOOH>HCN>H2>ROH
Basic order: NOo−3<RCOOo−<CNo−<o−OH<ROo−
(V)<(III)<(IV)<(I)<(III)
Decreasing order of basically and hence β− elimination is: (II)>(I)>(IV)>(III)>(IV)
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Single Correct
Explore 161 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Single Correct Answer Type
Explore 26 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRYChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Multiple Correct
Explore 35 Videos
- APPENDIX INORGANIC VOLUME 2Book:CENGAGE CHEMISTRYChapter:APPENDIX INORGANIC VOLUME 2Exercise:Short Answer Type
Explore 179 Videos
- BIOMOLECULESBook:CENGAGE CHEMISTRYChapter:BIOMOLECULESExercise:Exercises Archives (Analytical And Descriptive)
Explore 8 Videos
Similar Questions
The decreaing order of the rate of niration of the following compounds is
I. Benzene, II. C6D6
III. Nitrobenzene, IV Chlorobenzene
View SolutionIn the reaction is:
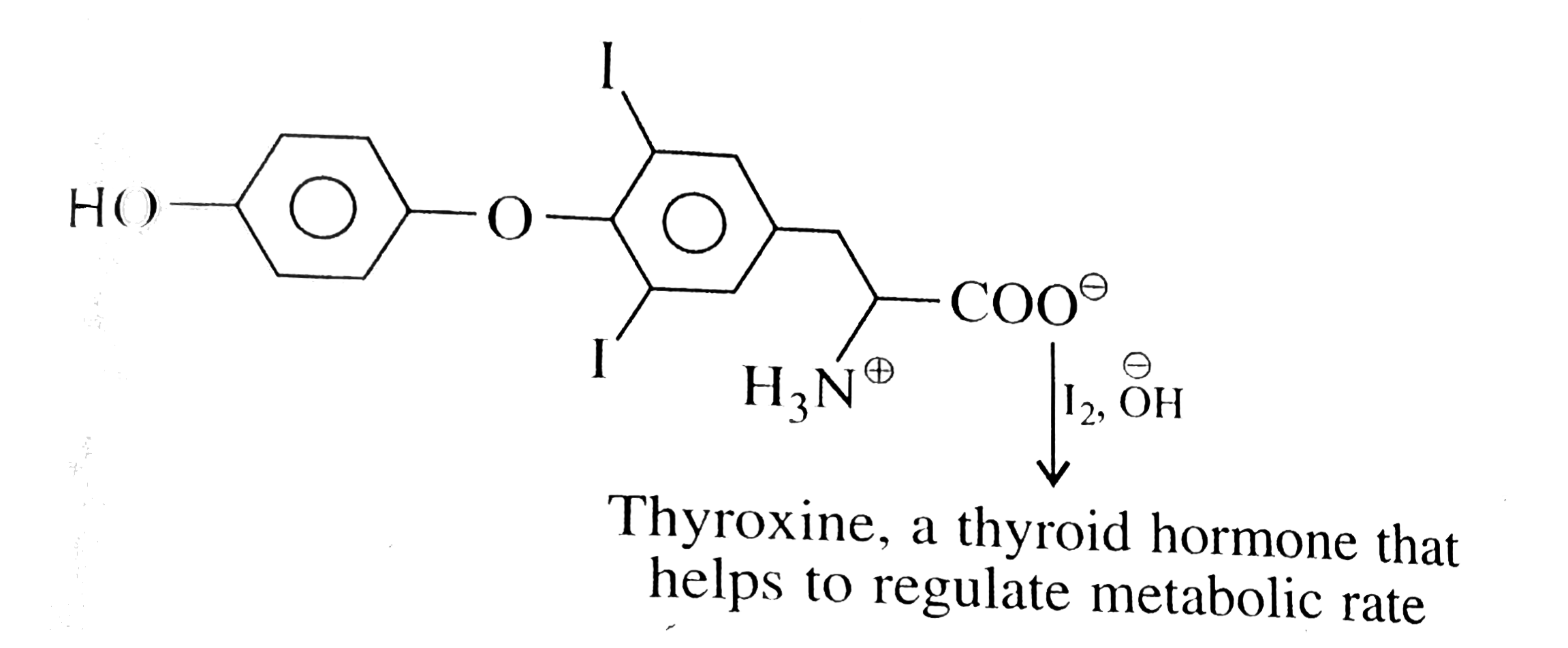
Thyoxine is:
View SolutionBoth t−butyl and (−SO3H) groups are used as a blocking group in certain synthesis of organic compounds. Which of the following statements are correct?
View SolutionWhich metals are generally extracted by the electrolytic processes ? What positions these metals generally occupy in the periodic table?
View SolutionWrite the structure of alanine at pH=2andpH=10.
View SolutionThe decreasing basic order of the following is:
i. o−CH3, ii. o−NH2, iii. o−OH, iv. Fo−
View SolutionThe decreasing nucleophilic oder of the following compounds is:
i. o−CH3, ii. o−NH2, iii. o−OH, iv. Fo−
View SolutionThe deecreasing leaving group order of the following compounds is: i. overset(o-)(C)H_(3) , ii. overset(o-)(N)H_(2) , iii. overset(o-)(O)H , iv. F^(o-)
View SolutionThe decreasing basic order of the following is: i. overset(o-)(C)H_(3) , ii. Overset(o-)(O)H , iii. CH_(3)COO^(o-) , iv. H_(2)O
View SolutionThe decreasing nucelophilic order of the following compounds is:
i. overset(o-)(C)H_(3),ii.Overset(o−)(O)H, iii. CH3COOo−, iv. H2O
View SolutionThe decrasing fugacity order of the following compounds is: i. overset(o-)(C)H_(3) , ii. Overset(o-)(O)H , iii. CH_(3)COO^(o-) , iv. H_(2)O
View SolutionThe decreasing basic order of the following is:
i. PhSOo−3, ii. C2H5SOo−3, iii. C2H5COOo−, iv. o−CN, v. o−OH
View SolutionThe decreaing basic order of the following is: i. overset(o-)(C)N , ii. overset(o-)(O)H , iii. overset(o-)(O) Me , iv. overset(o-)(C)H_(3) , v. H^(o-)
View SolutionThe decreasing nucelophilc order of the following compounds is:
i. o−CN, ii. o−OH, iii. o−OMe, iv. o−CH3, v. Ho−
View SolutionThe decreasing fugacity order of the following is: i. overset(o-)(C)N , ii. overset(o-)(O)H , iii. overset(o-)(O) Me , iv. overset(o-)(C)H_(3) , v. H^(o-)
View SolutionThe correct order of reactivity of PhMgBr with Ph - overset(O)overset(||)(C ) - Ph, " " CH_(3) - overset(O)overset(||)(C ) - H, " " CH_(3) - overset(O)overset(||)(C ) - CH_(3) " (I) ""(II) ""(III) "
View Solution

