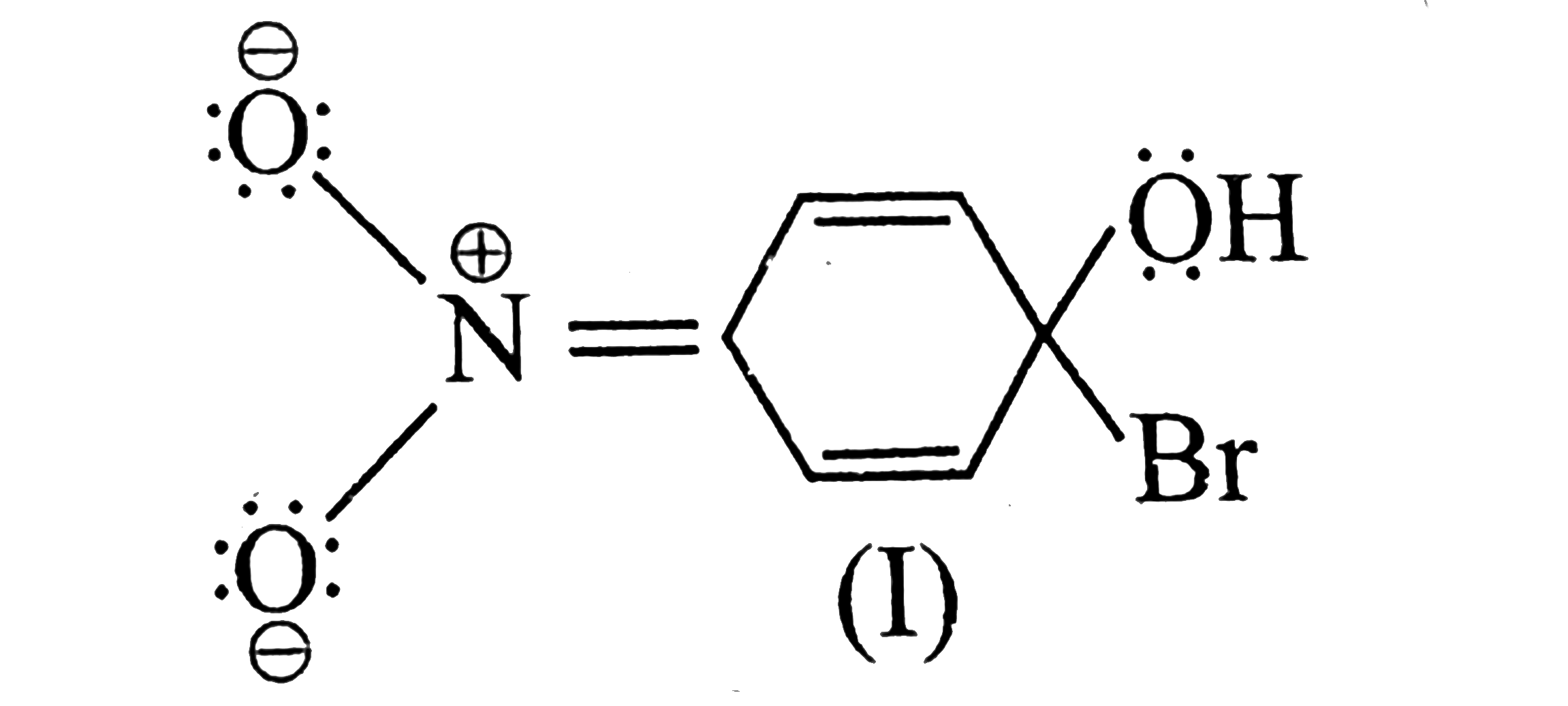
Which combination of recatns will not give species (I) shown as a reavitve intermedicate ?
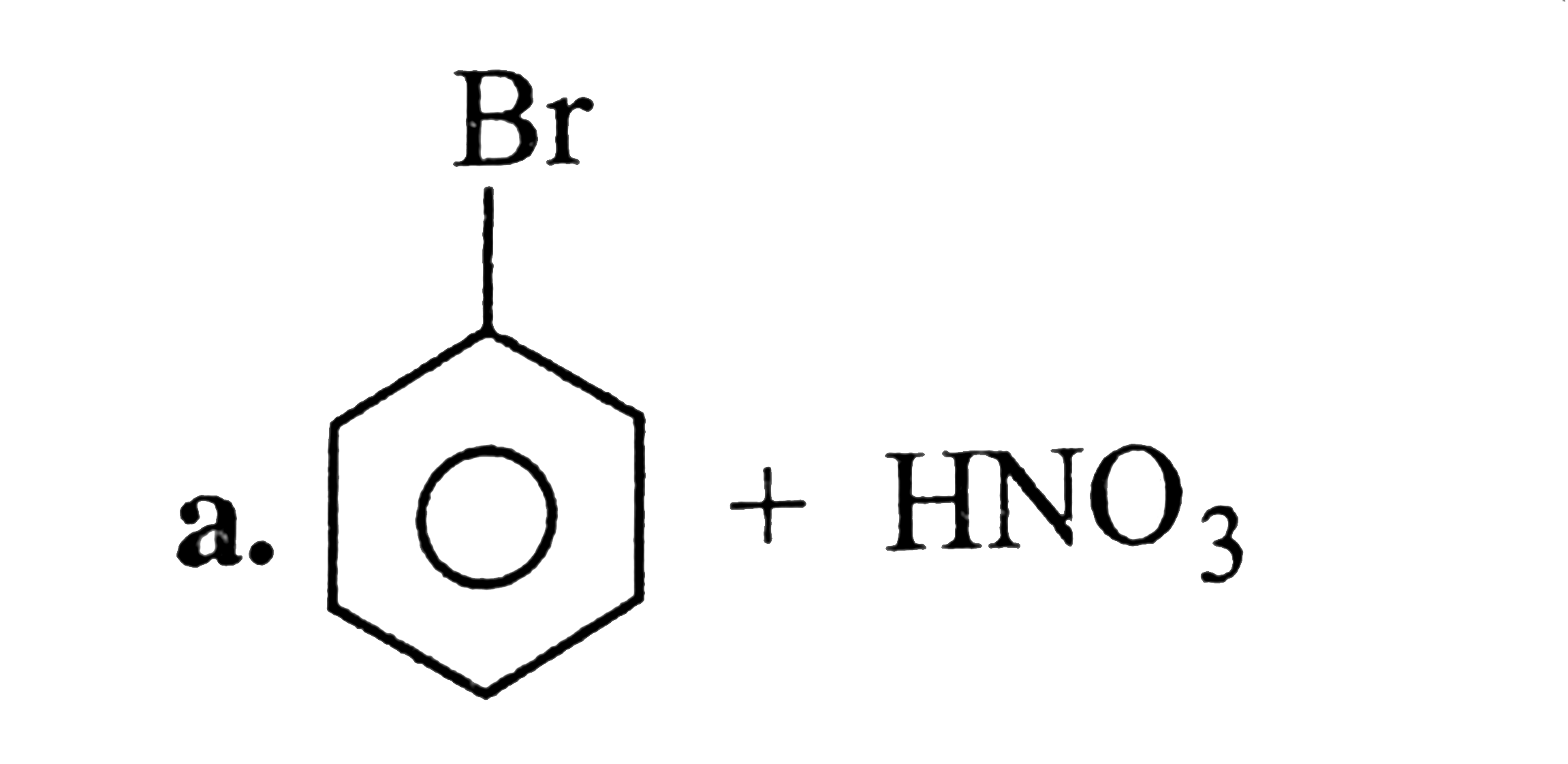
A
B
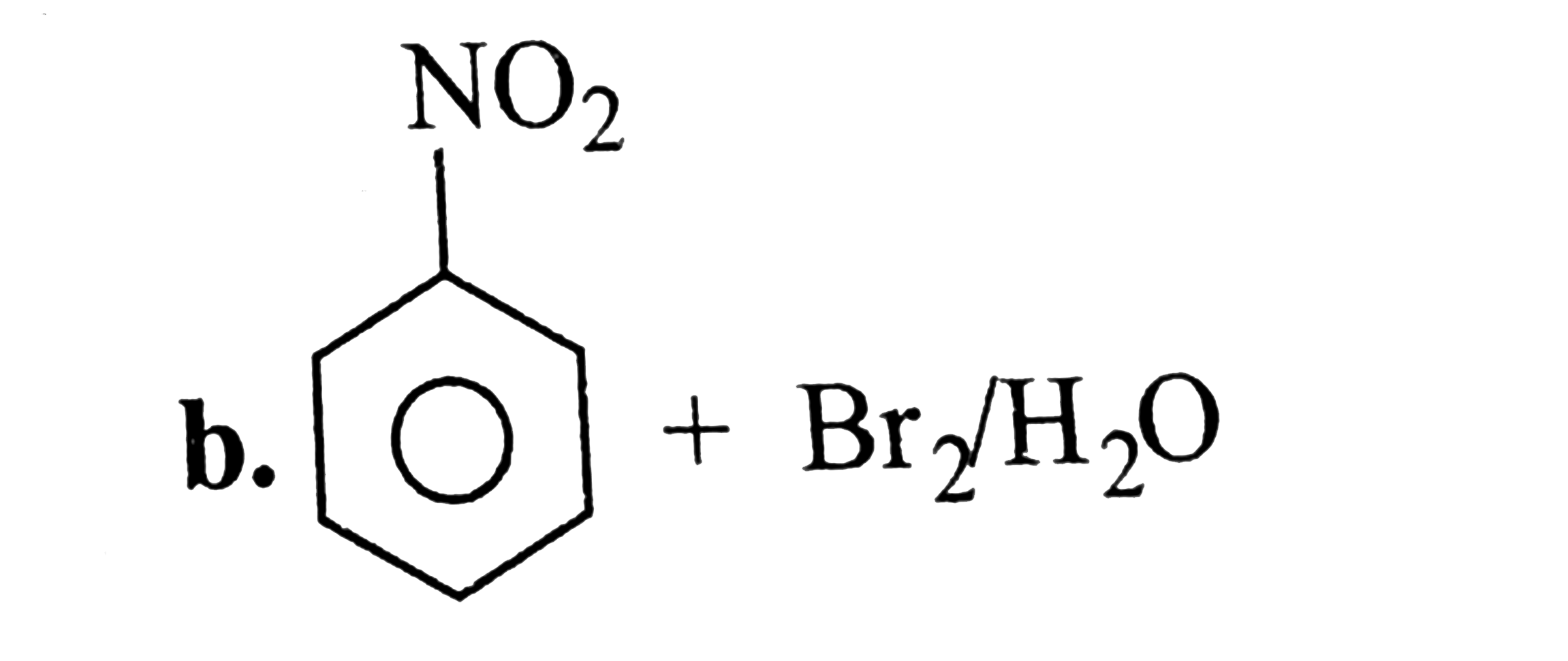
C
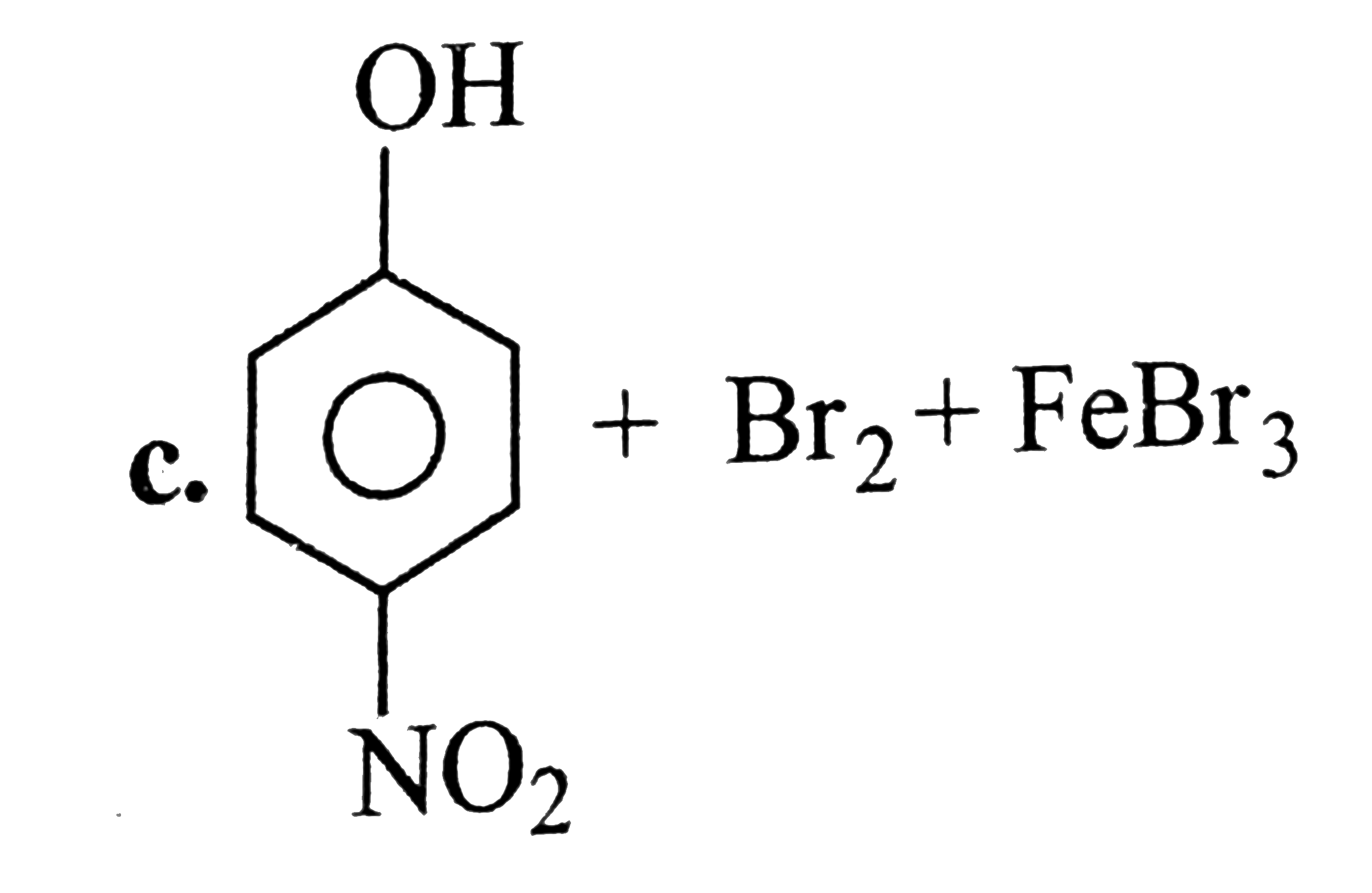
D
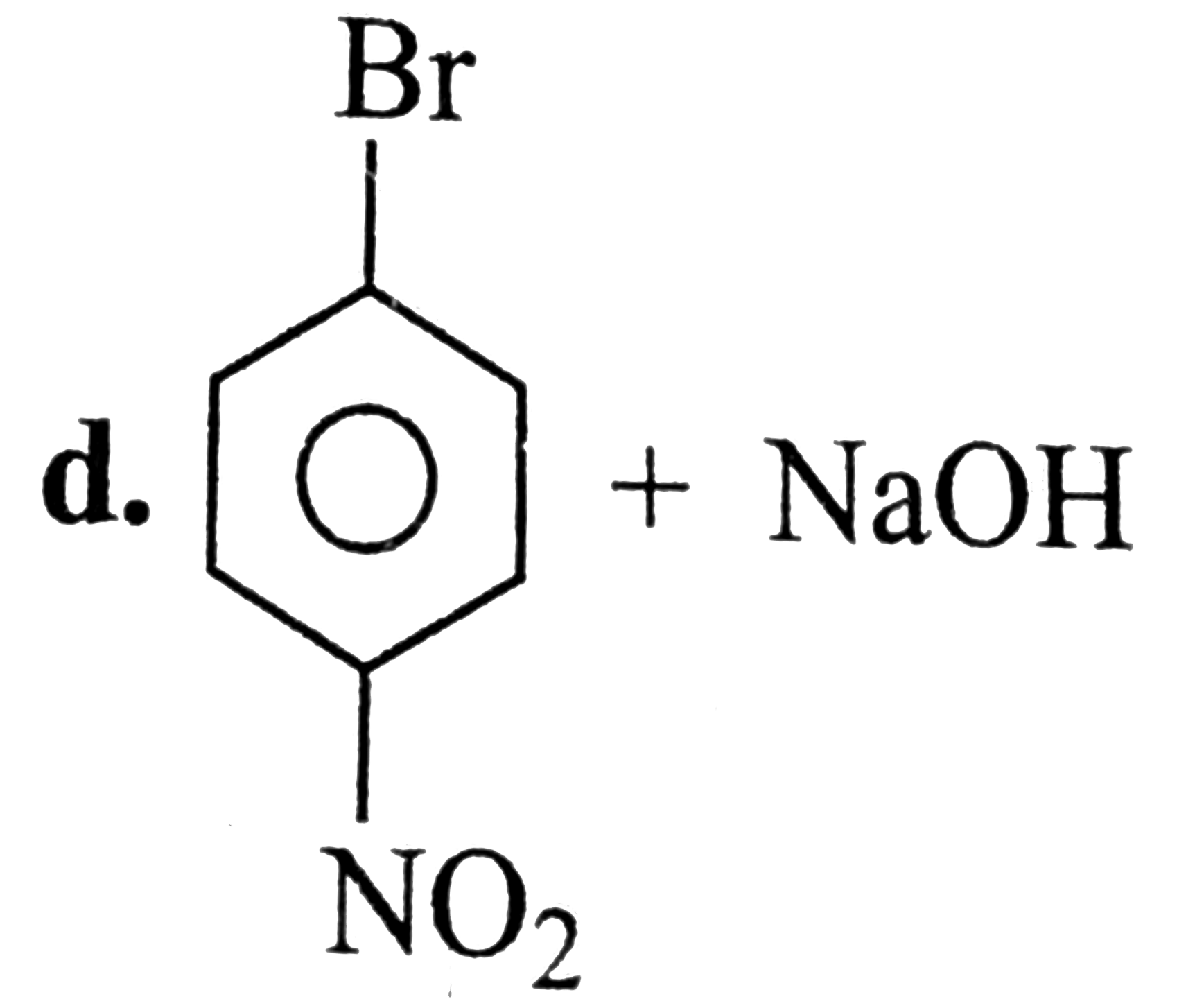
Text Solution
The correct Answer is:(a,b,c)
a. SE reactioln by (overser(⊕)(N)O2) species (I) is not formed. So (a) is correct answer.
b. SE reaction by Br⊕ will attack at m− postiton. Species (I) is not formed. So (b) is correct statemetns.
c. SE reaction by Br⊕,(−OH) group is o− and p− directing (class −OH decides orientations), p− position is blocked. Species (I) is not formed. So (c) is a correct statements.
d. ArSN reaction (addition -eliminiatiobn) reaction by o−OH nucleophile. Species (I) is formed.
So (d) is an incorrect statemetns.
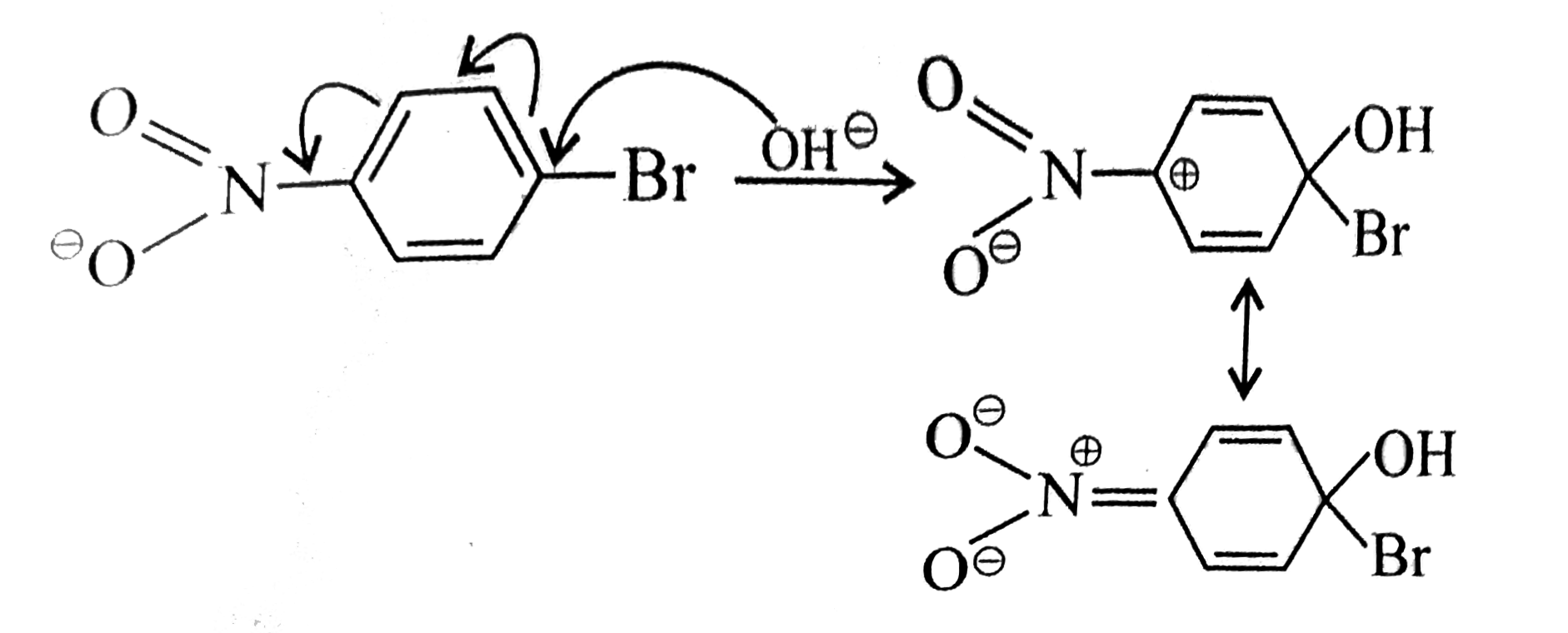
|
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Single Correct
161 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Single Correct Answer Type
26 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRYCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Multiple Correct
35 Videos
- APPENDIX INORGANIC VOLUME 2BOOK - CENGAGE CHEMISTRYCHAPTER - APPENDIX INORGANIC VOLUME 2EXERCISE - Short Answer Type
179 Videos
- BIOMOLECULESBOOK - CENGAGE CHEMISTRYCHAPTER - BIOMOLECULESEXERCISE - Exercises Archives (Analytical And Descriptive)
8 Videos
Similar Questions
Knowledge Check
Which combination will give methyl isocyanate ?
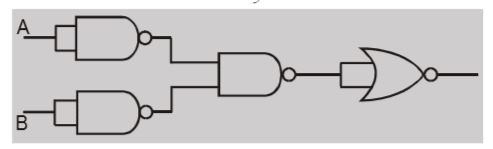
ACH3Cl+OBCH3NH2+CH2Cl2CCH3NH2+COCl2DC2H5OH+COCl.The combination of the gates shown will produce
_E01_427_Q01.png) AOR gateBAND gateCNOR gateDNAND gate
AOR gateBAND gateCNOR gateDNAND gateThe combination of the gates shown will produce
_E01_428_Q01.png) AOR gateBAND gateCNOR gateDNAND
AOR gateBAND gateCNOR gateDNAND
The combination of the gates shown will produce
_E01_428_Q01.png)
View SolutionThe combination of the gates shown represents : -
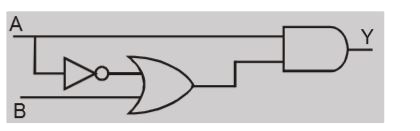
View SolutionThe combination of the gates shown represents :-
_E01_431_Q01.png)
View SolutionThe combination of the gates shown below produces
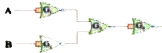
View SolutionThe combination of the gates shown represents :-
_E01_431_Q01.png)
View Solution