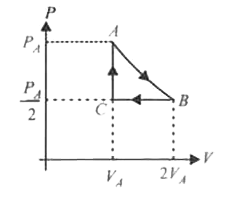
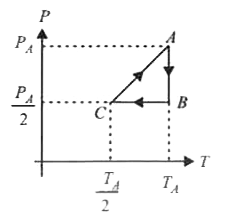
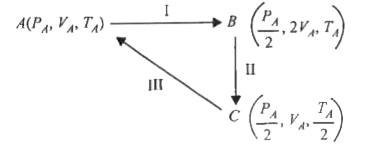
Three moles of an ideal gas Cp=7/2R at pressure PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally the gas is compressed at constant volume to the original pressure PA.The correct P-V and P-T diagram indicating the process are
The correct Answer is:a, c
Let the process starts from initial pressure PA, volume VAand temperature TA.
I. Isothermal expansion (PV = constant) at temperature TAto twice the initial volume VA II. Compression at constant pressure PA/2 to original volume VA(i.e.,V∝T)
III. Isochoric process (at volume VA) to initial conditions (i.e.,P∝T).
Topper's Solved these Questions
- THERMODYNAMICSBook:MTG-WBJEEChapter:THERMODYNAMICSExercise:WB JEE Previous Years Questions
Explore 12 Videos
- THERMODYNAMICSBook:MTG-WBJEEChapter:THERMODYNAMICSExercise:WB JEE WORKOUT ( CATEGORY 2 : Single Option Correct Type (2 Mark )
Explore 15 Videos
- SOLID STATE ELECTRONS Book:MTG-WBJEEChapter:SOLID STATE ELECTRONS Exercise:WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type)
Explore 15 Videos
- WAVE OPTICSBook:MTG-WBJEEChapter:WAVE OPTICSExercise:WB JEE PREVIOUS YEARS QUESTION (MCQ.s)
Explore 9 Videos
Similar Questions
Three moles of an ideal gas (Cp=7/2R) at pressure pA and temperature TA is isothermally expanded to twice its initial volume. It is then composed at constant pressure to its original volume. Finally the gas is compressed at constant volume to its original pressure pA (i) Sketch p−V and p−T diagrams for the complete process (b) Calculate the net work done by the gas and net heat supplied to the gas a during the complete process.
View SolutionThree moles of an ideal gas (Cp=72R) at pressure, PA and temperature TA is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure PA.
(a) Sketch P-V and P-T diagrams for the complete process.
(b) Calculate the net work done by the gas, and net heat supplied to the gas during the complete process.
View Solution
Knowledge Check
One mole of an ideal gas at pressure P0 and temperature T0 is expanded isothermally to twice ist volume and then compressed at constant pressure to (V0/2) and the gas is brought bac to original state by a process in which PαV (pressure is direclty proportional to volume). The correct temperature of the process is
A B
B C
C D
D
A gas is suddenly compressed to one fourth of its original volume. What will be its final pressure, if its initial pressure is p
ALess then pBMoer then PCpDEither(a) or ©A monatomic gas is compressed adiabatically to 1(4)th of its original volume , the final pressure of gas in terms of initial pressure P is
A7.08 PB8.08 PC9.08 PD10.08 P
A sample of an ideal gas has pressure p0, volume V0 and tempreture T0. It is isothermally expanded to twice its oringinal volume.it is then compressed at constant pressure to have the original volume V0. Finally, the gas is heated at constant volume to get the original tempreture.(a) show the process in a V-T diagram (b) calculate the heat absorbed in the process.
View SolutionThree moles of an ideal gas (Cp=72R) at pressure p0 and temperature T0 is isothermally expanded to twice its initial volume. It is then compressed at a constant pressure to its original volume.
(a) Sketch p-V and p-T diagram for complete process.
(b) Calculate net work done by the gas.
(c) Calculate net heat supplied to the gas during complete process.
(Write your answer in terms of gas constant =R)
View SolutionAn ideal gas intially has pressure P volume V and temperature T. Its is isothermally expanded to four times of its original volume, then it is compressed at constant pressure to attain its original volume V. Finally, the gas is heated at constant volume to get the original temperature T. (a) Draw V−T curve (b) Calculate the total work done by the gas in the process.(given ln2=0.693)
View SolutionOne gram mole of an ideal gas at N.T.P is first expanded isothermally to twice the origional volume. It is then compressed at constant volume, till its pressure is raised to the original value. Calculate the total amount of work done. Given R=8.3mole_1K−1.
View SolutionA monatomic gas is compressed adiabatically to 1(4)th of its original volume , the final pressure of gas in terms of initial pressure P is
View Solution

