When rate is plotted vs (a−xn), a straight line is obtained with positive slope, passing through orgin. Draw the graphs and discuss.Solution in Telugu
Step by step video & image solution for When rate is plotted vs (a -x^(n)), a straight line is obtained with positive slope, passing through orgin. Draw the graphs and discuss. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETCSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETCSEXERCISE - EXERCISE -3.1.3
6 Videos
- CHEMICAL EQUILIBRIUMBOOK - AAKASH SERIESCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS
5 Videos
- CHEMICAL KINETICSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETICSEXERCISE - Objective Exercise - 4 (Assertion (A) & Reason (R) Type Questions)
44 Videos
Similar Questions
What is activated complex ? Discuss its formation and transformation .
View SolutionThe values of Kp1andKp2 for the reaction given below are in the ratio 2: 1. If degree of dissociation of X and A are equal, then total pressure of equilibrium (1) and cquilibrium (2) are in the ratio
X⇔Y+Z,Kp1:A⇔2B:Kp2
View Solution(A) Spontaneous reaction may be slow or fast.
(R ) Spontaneous nature deals with feasibility of the reaction but not rate.
View SolutionIf a graph is plotted between log(a−x) and t, the slope of the straight line is equal to −0.03. The specific reaction rate will be
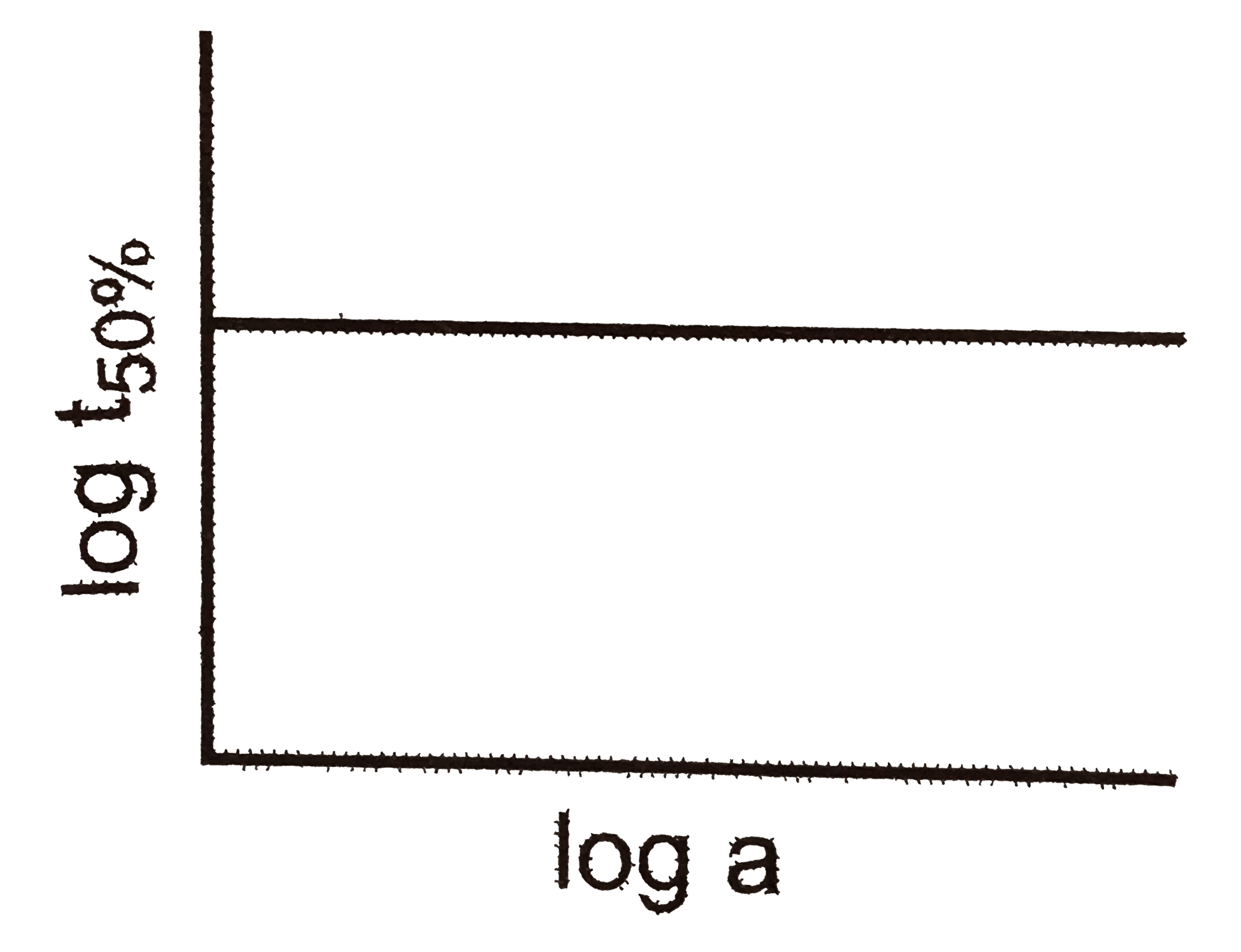
View SolutionA graph plotted between log t50% vs log concentration is a straight line. What conclusion can you draw from this graph?
View SolutionAccording to Moseley, a straight line graph is obtained on plotting:
View SolutionA graph between time (t) and the substance consumed at any time is found to be a straight line passing through the orginal This indicates that the reaction is of :
View SolutionThe straifht line graph with negative slope for integrated rate equation , kt =In[A]0−In[A]t is obtained by plotting a graph of _____.
View Solutionप्रथम कोटि अभिक्रिया के लिए Log k एवं 1/T में ग्राफ खींचते हैं, तो एक सरल रेखा प्राप्त होती है। प्राप्त रेखा की प्रवणता (ढाल) होगा
View Solutionlogxn तथा log p के मध्य ग्राफ खींचने पर सीधी रेखा पाप्त होती है जिसका ढाल किसके तुल्य होगा-
View SolutionFor a zero order reaction, (A) plot of [R_(0)] vs time is a straight line (B) plot of [R] vs time is a straight line (C) plot of [R] vs time has slope =k (D) plot of [R_(0)] vs time has slope =-k
View SolutionFor a 1st order reaction log(a−x) is plotted Vs time, a straight line is obtained with slope
View SolutionWhen for a reaction log (a-x) is plotted Vs time a straight line is obtained, with slope −0.03s−1. Find the rate constant of the reaction.
View SolutionWhen rate of a 1st order reaction is plotted Vs. concentration of the reactant a straight line is obtained, whose slope is equal to____
View SolutionA plot of log[R0]R vs time is a straight line passing through origin point. What is order of the reaction?
View Solution

