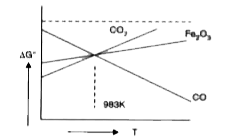
Draw Ellingham diagram [ΔG∘VsT] for the oxidation of carbon monoxide and for the formation of Fe2O3 and CO. Suggest suitable reducing agents for the reduction of Fe2O3 at a temperature below 983 K (710∘C) and at a temperature above 983 K. Solution in Kannada
Ellingham diagram for CO
Ellingham diagram for CO2 and Fe2O3
Below 983K - CO
Above 983K - C
Similar Questions
Correct statements form the graph
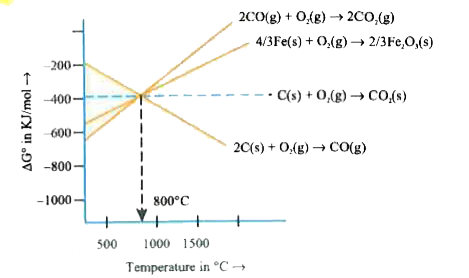
I) Above 1073K, ΔG0 for the formation of Fe2O3 is less negative than ΔG0 for the formation of CO from carbon
II) Above 1073K, Carbon can reduce Fe2O3
III) Below 1073K, CO can reduce Fe2O3
IV) In blast furnace, reduction of Fe2O3 occurs in different temperature ranges with below 1073K by CO (or) above 1073K by carbon
View SolutionCarbon monoxide is more effective reducing agent than carbon below 983 k but, above this temperature, the revere is true -Explain.
View SolutionCarbon can reduce ferric oxide to iron at a temperature above 983 K because
View SolutionCO is more effective reducing agent than coke below 983 K, but above 983 K the reverse is true. Why?
View SolutionWhich is better reducing agent at temperature 983 K, C or Co?
View SolutionAmong carbon and carbon monoxide which one is a better reducing agent for Fe2O3, above 1000 K?
View SolutionCarbon cannot reduce Fe_2O_3 to Fe at a temperature below 983 K because
View Solution

