Define energy of activation. Draw a diagram of energy profile to show the influence of a positive catalyst on the energy of activation of a reaction.Solution in Kannada
Video Solution
Text Solution
Minimum extra energy that the reacting molecule should acquire to reach threshold energy/ enter into effective collision and to from products.
Or
it is the difference between threshold energy and normal average energy of the reacting molecule.
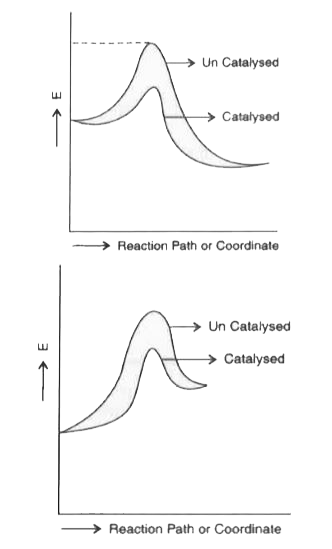
|
Similar Questions
Define 'activation energy' of a reaction.
View SolutionDefine activation energy of a reaction.
View SolutionDefine activation energy of a reaction.
View SolutionDraw a graph of potential energy V/S reaction co - ordinates showing the effect of catalyst on activation energy (E_(a)) of a reaction.
View SolutionDefine activation energy of a reaction.
View SolutionDerive the rate equation for 1st order reaction. What is activation energy? Draw an energy profile diagram for exothermic reaction.
View Solutionउत्प्रेरक की उपस्थिति में अभिक्रिया की सक्रियण ऊर्जा .........जाती है।
View Solution

