For a first order reaction A(g)⇔3B(g) the concentration verses time graph is given below:
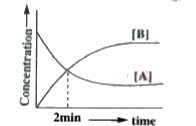
What is the half life in minutes (answer to nearest intetger)?Solution in Telugu
The correct Answer is:5
A⇔3B
A0 0
(A0−x) 3x
(3A04) 3A04
Here A0/4,K×t=2.303log(C0C1),K×2=2.303×lot(A03A0/4)
K=2.3032×log(43)=2.3032×(log4−log3)=2.3032×0.1249
(t12)=0.693K=0.693(2.303×0.12492),t12=(0.3010×20.1249)=4.8198.t12≅5
Similar Questions
For a first order reaction, if ‘a’ is the initial concentration of reactant, then the half life time is
View Solutionअभिक्रिया X(g)→Y(g)+Z(g) के लिये अर्द्ध-आयुकाल 10 मिनट है कितने समय में X की सान्द्रता इसकी मूल सान्द्रता से 10% रह जायेगी
View SolutionFor a first order reaction A_((g))hArr3B(g) the concentration verses time graph is given below What is the half life in minutes (answer to the nearest integer)?
View Solutionप्रथम कोटि अभिक्रिया में, अभिकारक का सान्द्रण 298 K पर 75 मिनट में प्रारंभिक सान्द्रण का 1/8 तक कम हो जाता है। मिनट में अभिक्रिया का अर्द्ध-आयु काल क्या है?
View SolutionThe half life of a first order reaction is 10 years . The time taken by 10 g of the reactant to reduce its half is ______.
View SolutionFor the first order reaction half-life time is ………….. on the initial concentration.
View SolutionHalf life time for first order reaction is 25 minutes . What time will be required for 99% completion of reaction ?
View Solution

