Which of the following statements are correct?Solution in Telugu
Time required for 75% completion is 1.5 times of half life for zero order reaction.
Time needed for a definite fraction of 1st order reaction not vary with the initial concentration.
Time for 25% reaction is one third of half life in second order process
Rate of 1st order reaction gets doubled if the conc of the reactant in increased to a two fold value.
The correct Answer is:A, B, C, D
C0−Ct=K×t,C0/2C0−C0/4=K×t12K×t=t=32×t12K×t12K×t=⎛⎜
⎜
⎜
⎜
⎜
⎜⎝1C0/2−1C0(13C04)−1C0⎞⎟
⎟
⎟
⎟
⎟
⎟⎠,
t12t=1C0×3C04−3=3C0C0×1=3,t=13×t12
Topper's Solved these Questions
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:PRACTICE SHEET-2 (LINKED COMPRHENSION TYPE QUESTIONS)
Explore 6 Videos
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:PRACTICE SHEET-2 (MATCH THE FOLLOWING QUESTIONS)
Explore 2 Videos
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:PRACTICE SHEET-1 (INTEGER ANSWER TYPE QUESTIONS)
Explore 6 Videos
- CHEMICAL KINETCSBook:AAKASH SERIESChapter:CHEMICAL KINETCSExercise:EXERCISE - 3.2
Explore 45 Videos
- CHEMICAL THERMODYNAMICSBook:AAKASH SERIESChapter:CHEMICAL THERMODYNAMICSExercise:Additional Practice Exercise
Explore 54 Videos
Similar Questions
Elemantary unimolecular reactions have first order rate laws, elementary bimolecular reactions have second order rate laws. A rate law is often derived from a proposed mechanism by imposing the state approximation or by assuming that there is a pre -equilibrium. A proposed mechanism must be consistent with the experiment rate law.
The decomposition of O3 obeys the mechanism give below
Step 1: O3⇔O2+(O) (both forward and backward reactions are fast)
Step 2: O3+(O)slow−−→O2+O2 (ignore) backward reaction), the rate of reaction is given by
View SolutionMatch the following columns
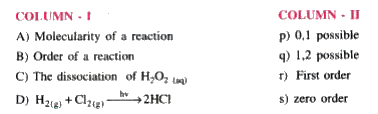
View SolutionIn a gaseous phase reaction A2(g)→B(t)+1(1/2)C(g), the increase in pressure from 100 mm to 120 mm is noticed in 5 minute.The rate of disappearance of A2 in mm min−1 is
View SolutionDiscuss on the kinetic spontaneity of a chemical reaction. Is a slow reaction spontaneous? Why?
View SolutionWhich among the following is not an exact differential?
View SolutionWhich of the following statement is (are) correct ?
View SolutionWhich of the following statements are correct statements ?
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following is/are correct statement/statements ?
View Solutionनिम्नलिखित कथनों में कौन-सा कथन सही नहीं है-
View Solutionনীচের কোন বক্তব্যটি সঠিক
View Solutionनिम्न में से कौनसा कथन सही नहीं है
View Solutionनिम्न में से कौन-सा कथन सही नहीं है?
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following statements is/are correct ?
View Solution
