The volume of perhydrol required to get one liter of oxygen at STP isSolution in Telugu
20 ml
10 ml
33.3ml
6 ml
The correct Answer is:B
Perhydrol is 30%(WV) i.e 100 Vl 1mL of 100 Vol H2O2→100mLO2
Topper's Solved these Questions
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:LECTURE SHEET EXERCISE -I (LEVEL-II (ADVANCED) More than one correct answer Type Questions)
Explore 5 Videos
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:LECTURE SHEET EXERCISE -I (LEVEL-II (ADVANCED) Linked Comprehension Type Questions)
Explore 2 Videos
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:QUESTIONS FOR DESCRIPTIVE ANSWERS
Explore 20 Videos
- HYDROCARBONSBook:AAKASH SERIESChapter:HYDROCARBONSExercise:ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))
Explore 5 Videos
- IONIC EQUILIBRIUM Book:AAKASH SERIESChapter:IONIC EQUILIBRIUM Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))
Explore 8 Videos
Similar Questions
In which of the following property, hydrogen does not resemble with halogen
View SolutionNormal water is mainly protium oxide, H2O However, if protium atoms in normal water molecule are replaced completely by deuterium atoms, the resulting water is called heavy water.
The ionization constant of protum water (H2O⇔H++OH)is1×10−14 and that in heavy water (D2O⇔OD−)is3×10−15is3×10−15H2O dissociates about
View SolutionWhy the use of hard water is disadvantages?
View SolutionMatch the intermediates formed in the reaction.
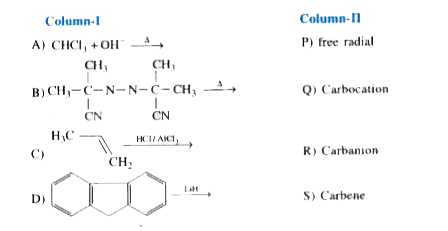
View SolutionA certain weak acid has Ka,=10−5. If the equilibrium constant for is its reaction with a strong base is repesented as y×10y then find the value of y.
View SolutionThe volume of perhydrol which on decompostion gives 1L of O_(@) at STP is
View SolutionThe volume of oxygen at STP required to burn 2.4 g of carbon completely is :
View Solution400 मिली मेथेन का पूर्ण दहन करने के लिए S.T.P. पर ऑक्सीजन के आवश्यक आयतन की गणना कीजिए।
View Solutionएक लीटर ऑक्सीजन 2 लीटर कार्बन मोनोऑक्साइड से क्रिया करके कार्बन डाइऑक्साइड बनाती हैं। S.T.P. पर होने वाली इस क्रिया में प्राप्त कार्बन डाइऑक्साइड का आयतन ज्ञात कीजिए।
View Solutionदो आयतन CO एक आयतन O2 से संयोग करके दो आयतन CO2 बनाती है।
2CO+O2→2CO2
50 लीटर CO2 प्राप्त करने के लिए कितने लीटर ऑक्सीजन की आवश्यकता होगी ?
View Solutionएक आयतन H2, एक आयतन क्लोरीन से संयोग करके दो आयतन HCl बनाती है। 10 लीटर हाइड्रोजन क्लोराइड गैस प्राप्त करने के लिए कितने हाइड्रोजन की आवश्यकता होगी ?
View SolutionFind out the volume (in ml) of perhydrol to be decomposed to get oxygen gas which is sufficient for combustion of 2.8 g of ethylene gas
View SolutionThe volume of perhydrol required to get one liter of oxygen at STP is
View SolutionThe volume of perhydrol which on decomposition gives 2 lit ofO2 gas at STP is
View SolutionThe volume of oxygen required at STP for the complete combustion of 2.2 g of propane
View Solution
