Degree of liness of water is expressed in terms of ppm of Solution in Telugu
CaO
CaCl2
CaCO3
Ca(HCO3)2
The correct Answer is:C
Degree of hardness of water is expressed in terms of equivalents of CaCO3
Topper's Solved these Questions
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - LECTURE SHEET EXERCISE -I (LEVEL-II (ADVANCED) More than one correct answer Type Questions)
5 Videos
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - LECTURE SHEET EXERCISE -I (LEVEL-II (ADVANCED) Linked Comprehension Type Questions)
2 Videos
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - QUESTIONS FOR DESCRIPTIVE ANSWERS
20 Videos
- HYDROCARBONSBOOK - AAKASH SERIESCHAPTER - HYDROCARBONSEXERCISE - ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))
5 Videos
- IONIC EQUILIBRIUM BOOK - AAKASH SERIESCHAPTER - IONIC EQUILIBRIUM EXERCISE - ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))
8 Videos
Similar Questions
In which of the following property, hydrogen does not resemble with halogen
View SolutionNormal water is mainly protium oxide, H2O However, if protium atoms in normal water molecule are replaced completely by deuterium atoms, the resulting water is called heavy water.
The ionization constant of protum water (H2O⇔H++OH)is1×10−14 and that in heavy water (D2O⇔OD−)is3×10−15is3×10−15H2O dissociates about
View SolutionWhy the use of hard water is disadvantages?
View SolutionMatch the intermediates formed in the reaction.
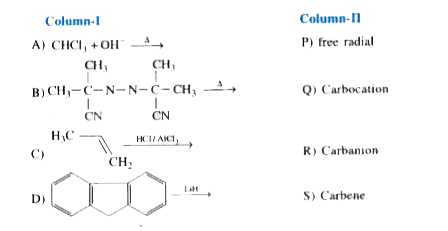
View SolutionA certain weak acid has Ka,=10−5. If the equilibrium constant for is its reaction with a strong base is repesented as y×10y then find the value of y.
View SolutionAn algebraic expression consisting of unlike terms of the same degree
View SolutionA sample of hard water contains 1 mg CaCl_2 and 1 mg MgCl_2 per litre. Calculate the hardness of water in terms of CaCO_3 present in per 10^(6) parts of water. (a). 2.5 ppm (b). 1.95 ppm (c). 2.15 ppm (d). 195 ppm
View SolutionDetermine the degree of hardness of a sample of water containing 30 ppm of MgSO4.
View SolutionDetermine degree of hardness in term of ppm of CaCO3 of 10−3 molar MgSO4 (aq).
View SolutionThe degree of hardness of water is usually expressed in terms of
View SolutionThe degree of hardness of water is usually expressed in terms of
View Solution1L of a sample of hard water contains 1 mg of each of CaCl_(2) and MgCl_(2) . Express the harnedd of the sample in ppm, in terms of CaCO_(3) .
View SolutionThe degree of hardness of water is usually expressed in terms of
View SolutionThe degree of hardness of water is usually expressed in terms of
View Solutionजल की कठोरता के मापन के लिए सामान्यतः प्रयुक्त होने वाले अभिकर्मक का नाम लिखिए ।
View Solution

