Discuss the shape of the BeF2 molecules using VSEPR model
Video Solution
Text Solution
The Lewis structure electron pair and the geometrical of BeF2 are
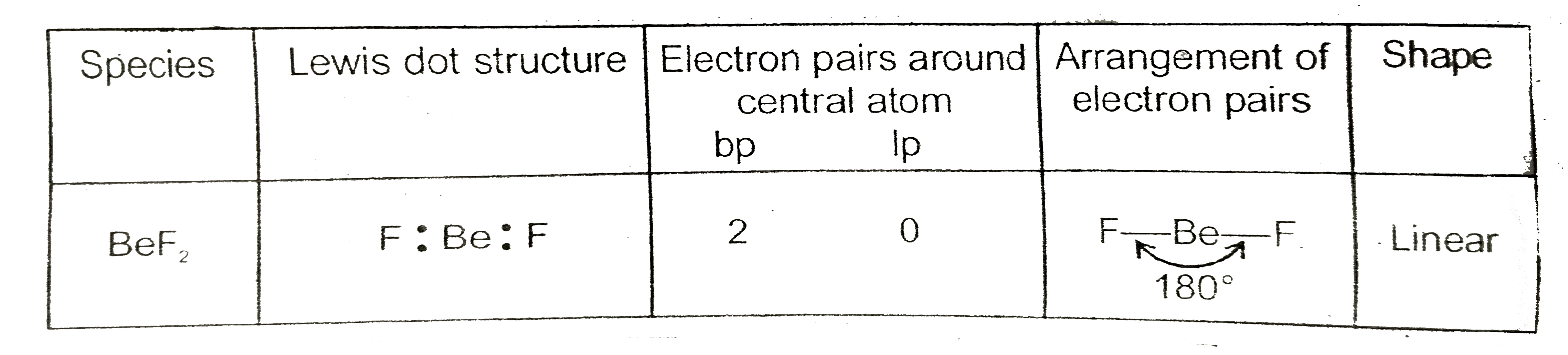
|
Similar Questions
Knowledge Check
The shape of XeF4 molecule according to VSEPR theory is
ASquare planarBSquare pyramidCTetrahedralDPyramidalFused BeF2 is
AionicBcovalentCboth ionic and covalentDa polymeric compound
Discuss the shapes of following molecules using VSEPR model :
(i) SiCl4 (ii) PH3 (iii) BeCl2
Which of these is/are polar molecules.
View SolutionDiscuss the shape of the following molecules using the VSEPR model:
BeCl2,BCl3,SiCl4,AsF5,H2S,PH3
View SolutionDiscuss the shape of the following molecules using the VSEPR model:
BeCl2,BCl3,SiCl4,AsF5,H2S,PH3
View SolutionDiscuss the shape of the IF5 using VSEPR model .
View SolutionDiscuss the shapes of the following molecules of the basis of VSEPR theory :
(i) CH4
(ii) PF5
(iii) NH3
(iv) H2O
(v) SF6.
View SolutionDiscuss the shape of CO2 molecule on the basis of hybridisation.
View Solution

