Discuss the shape of the BCl3 molecules using VSEPR model .
Video Solution
Text Solution
Lewis dot structure : :B..Cl:Cl,bp = 3 , lp = 0
Arrangement of electron pairs
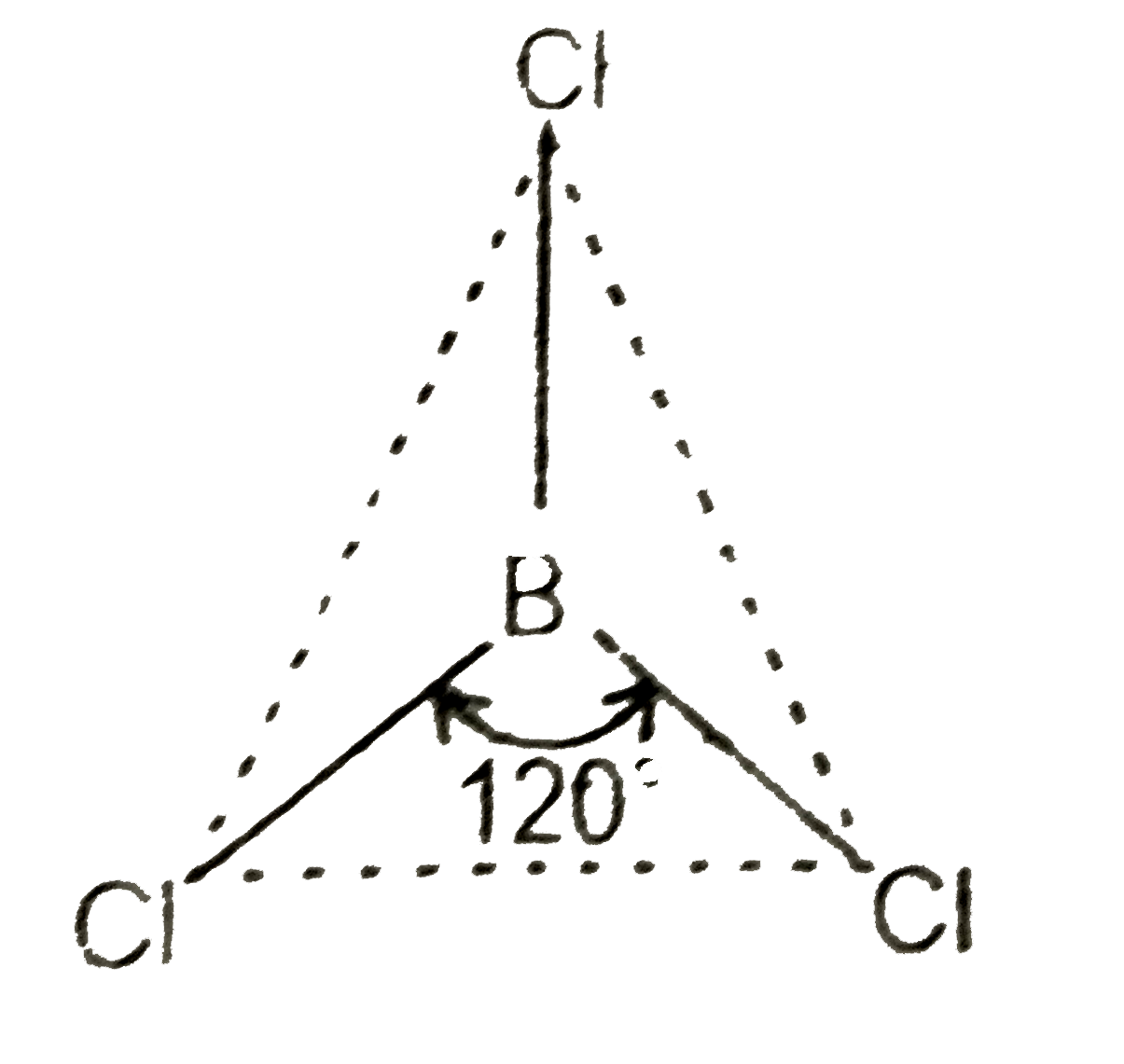
|
Similar Questions
Knowledge Check
The shape of SF3Cl3 molecule is:
Atrgonal bi-pyramidalBcubicCoctahedralDthetrahedral.The shape of the benzene3 molecule is
AtetrahedralBtrigonal planarCregular flat hexagonDoctahedralThe shape of the molecule SF2Cl2 is
Atrigonal bipyramidalBcubicCoctahedralDtetrahedral
Discuss the shape of the SiF4 molecule using VSEPR model .
View SolutionDiscuss the shape of the following molecules using the VSEPR model:
BeCl2,BCl3,SiCl4,AsF5,H2S,PH3
View SolutionDiscuss the shapes of following molecules using VSEPR model :
(i) SiCl4 (ii) PH3 (iii) BeCl2
Which of these is/are polar molecules.
View SolutionDiscuss the shape of the following molecules using the VSEPR model:
BeCl2,BCl3,SiCl4,AsF5,H2S,PH3
View SolutionThe shape of BF3 molecule is ______.
View Solution
