Study the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
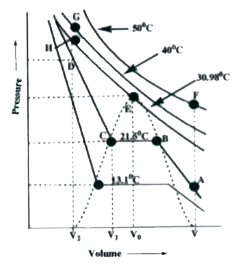
At 21.5°C decrease in volume(B-C) doesn't result into increase in pressure becauseSolution in Telugu
The compression causes increase in solidification
At point B only all gases convert into solid
The compression causes further increase in condensation
Carbon dioxide is real gas
B-C is the process of l→g
Similar Questions
Study the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
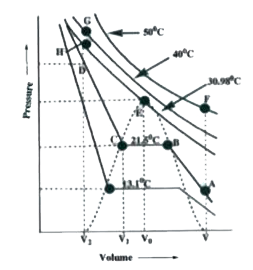
Carbondioxide present at F can be liquified
View SolutionStudy the following isotherms of carbondioxide at various temperature and answer the following questions based on that:
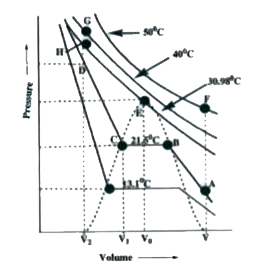
Choose the temperature above which CO2 cannot be liquified whatsoever applied pressure is
View SolutionThe temperature of a gas is increased by 1 aC. Then from the following statements pickout the correct one
1) The volume increases by 1/273 of its volume at 0°C at constant pressure
2) The pressure increases by 1/273 of its pressure at 0°C at constant volume
3) The volume decreases by 1/273 of its volume at 0°C
4) The pressure is doubled to its pressure at 0°C
View SolutionIn a isothermal process on an ideal gas, the pressure increases by 0.5% . The volume decreases by about.
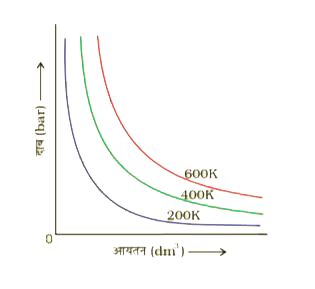
View Solutionविभिन्न तापमानों पर आयतन के साथ दाब में परिवर्तन को चित्र 5.3 में दिए ग्राफ द्वारा दर्शाया जा सकता है|
इस ग्राफ़ के आधार पर निम्नलिखित प्रश्नों के उत्तर दीजिए -
(i) स्थिर ताप पर, दाब बढ़ाने से गैस का आयतन किस प्रकार परिवर्तित होगा?
(ii) स्थिर दाब पर ताप को 200K से 400K बढ़ाने पर गैस का आयतन किस प्रकार परिवर्तित होगा?
View SolutionWhich of the following statements are related to Boyle's law?
a. When pressure increases volume decreases.
b. When temperature increasen, volume increases.
c. If P is pressure and V the volume N then P×V is a constant.
d. At constant temperature and pressure the volume of a gas is directly proportional to the number of molecules.
View SolutionIsotherms of carbon dioxide at various temperatures are represented in figure. Answer the following questions based on this figure. Will condensation take place when the temperature is T_(3) ?
View Solutionതാഴെപ്പറയുന്നവയിൽ ബോയിൽ നിയമവുമായി ബന്ധപ്പെട്ട പ്രസ്താവന ഏത്? a. മർദം കൂടുമ്പോൾ വ്യാപ്തം കുറയുന്നു. b. താപനില കൂടുമ്പോൾ വ്യാപ്തം കൂടുന്നു. c. മർദ്ദം P എന്നും വ്യാപ്തം V എന്നും സൂചിപ്പിച്ചാൽ PxV ഒരു സ്ഥിരസംഖ്യ ആയിരിക്കും. d. താപനില, മർദ്ദം ഇവ സ്ഥിരമായി ഇരിക്കുമ്പോൾ വാതകങ്ങളുടെ വ്യാപ്തം തന്മാത്രകളുടെ എണ്ണത്തിന് നേർ അനുപാതത്തിൽ ആയിരിക്കും.
View Solution

