Explain how does the -OH group attached to a carbon of benzene ring activate it towards electrophilic substitution ?
Text Solution
→ The -OH group is electron releasing groups. It increases the electron density at ortho and para positions through resonance. The electrophiles being electron deficient in nature attacks the ring at electron high density regions, i.e., ortho and para- positions. Thus, -OH group of benzene activates the ring towards electrophilic substitution reaction.

→ From the structures II, III and IV, it is clear that ortho and para positions are most active sites for the electrophilic attack.
|
Topper's Solved these Questions
- ALCOHOLS , PHENOLS AND ETHERSBOOK - KUMAR PRAKASHANCHAPTER - ALCOHOLS , PHENOLS AND ETHERSEXERCISE - SECTION-D (NCERT EXEMPLAR SOLUTION) (Multiple Choice Questions (MCQs))
16 Videos
- ALCOHOLS , PHENOLS AND ETHERSBOOK - KUMAR PRAKASHANCHAPTER - ALCOHOLS , PHENOLS AND ETHERSEXERCISE - SECTION-D (Multiple Choice Questions (MCQs) More than one options
5 Videos
- ALCOHOLS , PHENOLS AND ETHERSBOOK - KUMAR PRAKASHANCHAPTER - ALCOHOLS , PHENOLS AND ETHERSEXERCISE - SECTION-B (Intext Questions and Answer)
23 Videos
- BIOMOLECULESBOOK - KUMAR PRAKASHANCHAPTER - BIOMOLECULESEXERCISE - SECTION - E (MCQs asked in Board Exams)
61 Videos
Similar Questions
Knowledge Check
Which of the following substituents deactivates the benzene ring towards electrophilic substitution?
A−CHOB−NR2C−NHCOCH3D−NO2The function of which one of the following group on benzene nucleus activate it towards electrophilic substitution :
A−C≡NB−O||C−HC−O||C−ORD−O−O||C−RThe presence of which one of the following group on benzene nucleus activate it towards electrophilic substitution :
A−C≡NB−O∣∣C−HC−O∣∣C−ORD−O−O∣∣C−R
Explain how does the (−−−OH) group attached to a carbon of benzene ring activate it towards electophililc substitution.
View SolutionExplain how an OH group attached to a carbon in the benzene ring activates benzene towards electrophilic substitution.
View SolutionHow many of the following are activated towards electrophilic aromatic substitution reactions?
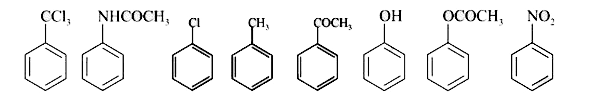
View SolutionHow many of the following are activated towards electrophilic aromatic substitution reactions?

View SolutionWhich of the following group activates the benzene ring most towards electrophilic substitution
View Solution

