Arrange water, ethanol and phenol in increasing order of acidity and give reason for your answer.
Text Solution
→ The acidic strength of a substance depends on the stability of the conjugate base. Stable is the conjugate base, stronger is the acid. The phenoxide ion is most stable among those listed because of resonance whereas in case of ethanol, there is no resonance and the +I effect of the ethyl group increase the electron density on the oxygen atom and destabilize the system. So, alcohol is weakest acid and phenol is the strongest acid. The order of acidic strength is: phenol>water>ethanol.
|
Similar Questions
Knowledge Check
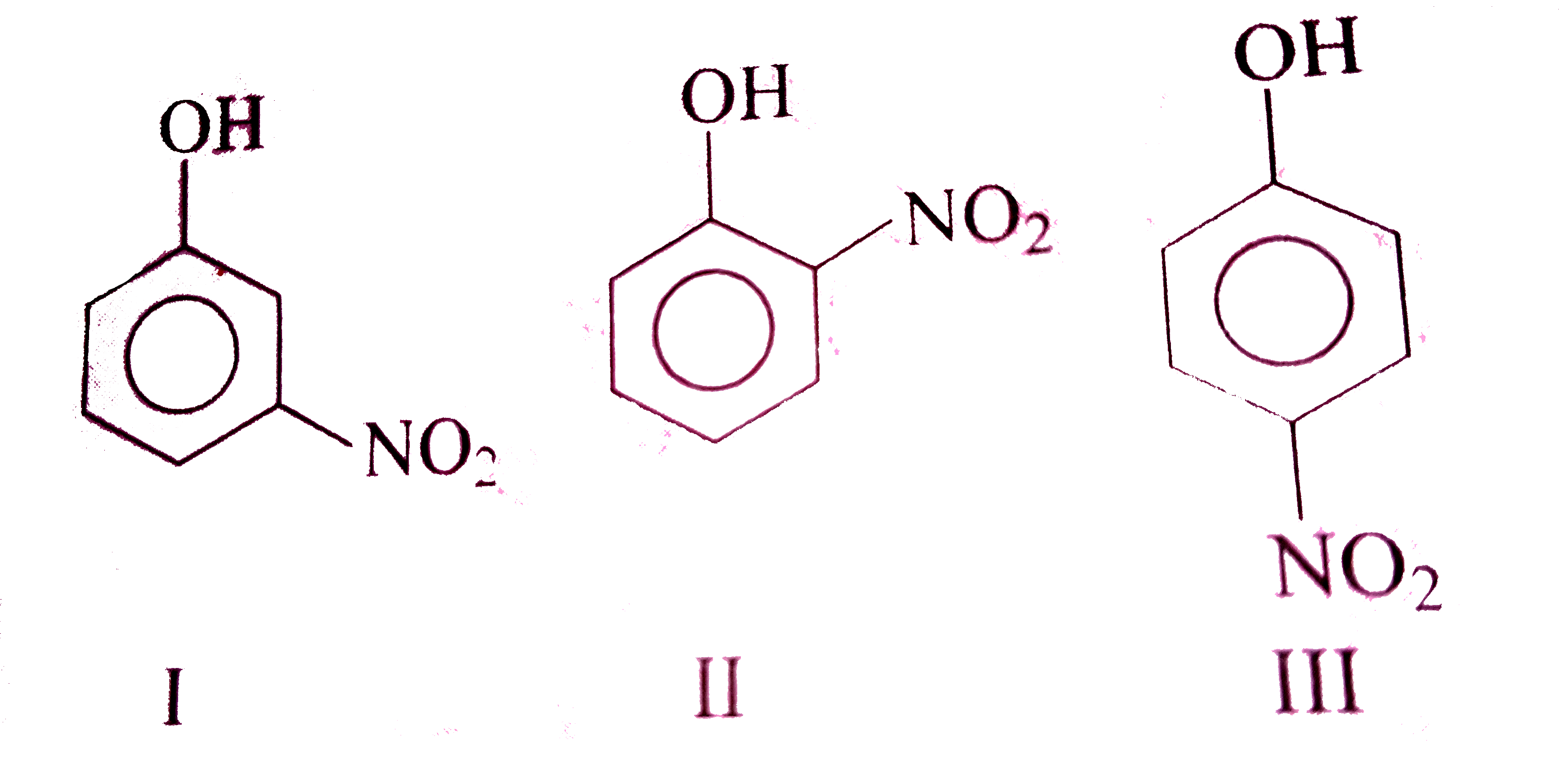
Arrange the above phenols in increasing order of Ka value.AI<II<IIIBIII<I<IICIII<II<IDI<III<II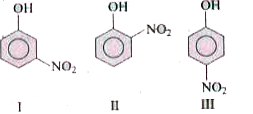
Arrange the above phenols in increasing order of Ka value.AI<II<IIIBIII<I<IICIII<II<IDI<III<II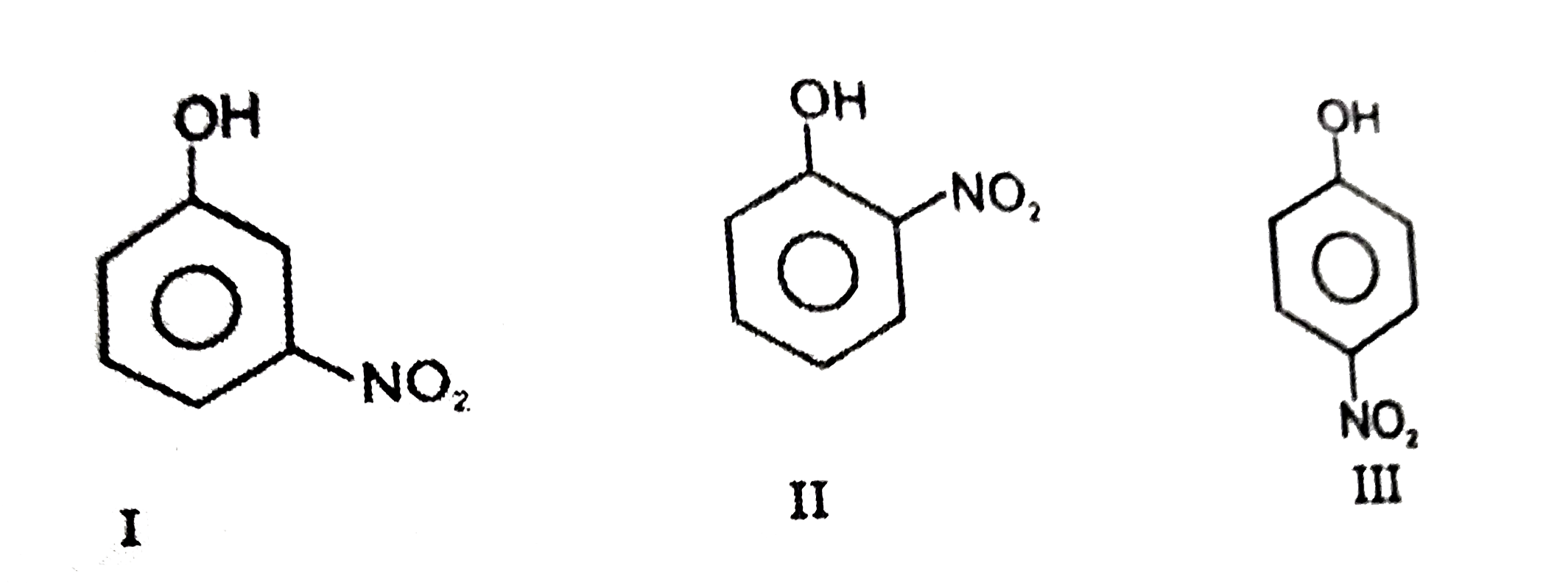
Arrange above phenol in increasing order of pKa value:AI<II<IIIBIII<I<IICIII<II<IDI<III<II
(a) Arrange the hydrides of group 16 in increasing order of the acidic character. Justify your answer. (b) Draw structure of XeOF4.
View Solution(a) Arrange the hydrides of group 16 in increasing order of the acidic character. Justify your answer. (b) Draw structure of XeOF4.
View SolutionArrange the following in the increasing order of acidity and explain your order.
Formic acid, acetic acid, floroacetic acid.
View SolutionArrange the following in the increasing order of acidity and explain your order.
Formic acid, acetic acid, chloroacetic acid.
View Solution
Arrange above phenol in increasing order of pKa value:
View Solution

