In Bohr model of atom :Solution in Telugu
the radius of nth orbit is proportional to n2
the total energy of the electron in the nth orbit is inversely proportional to n
the angular momentum of the electron in an orbit is an integral multiple of h2π
the magnitude of the potential energy of the electron in an orbit is greater than its kinetic energy
The correct Answer is:A, C, D
Step by step video, text & image solution for In Bohr model of atom : by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Practice sheet - 2 (Section - B : Linked comprehension Type questions))
Explore 5 Videos
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Practice sheet - 2 (Section -C : Matching/Straight objective type questions))
Explore 2 Videos
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Lecture Sheet - 2 (Section -C : Matching/Straight objective type questions))
Explore 2 Videos
- AROMATIC HYDROCARBONSBook:AAKASH SERIESChapter:AROMATIC HYDROCARBONSExercise:OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)
Explore 10 Videos
- CHEMICAL BONDINGBook:AAKASH SERIESChapter:CHEMICAL BONDINGExercise:OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )
Explore 39 Videos
Similar Questions
Atoms more complicated than hydrogen have more than one proton in their nucleus. Let Z stands for the number of protons in a nucleus. Also imagine that an atom loses all but one of its electrons so that it changes into a positively charged ion with just one electron. Bohr.s formula for the energy levels of the hydrogen atom can easily be changed to apply to such ions. It becomes Em=−Z2e4me8ε20h2n2, where m= mass of electron , e= change of electron, n = orbit number.
The ionization potential of Het in ground state is
View SolutionIf in Bohr.s model, for uni electronic atom following symbols are used :
rn,Z→ Radius of nth orbit with atomic number z
Un,Z→ Potential energy of e−
Kn,Z→ Kinetic energy of e−
Vn,Z→ Velocity of e−
Tn,Z→ Time period of revolution

View SolutionAccording to Bohr.s theory, En = total energy, Kn = kinetic energy , Vn = potential energy, rn = radius of nth orbit. Match the following.
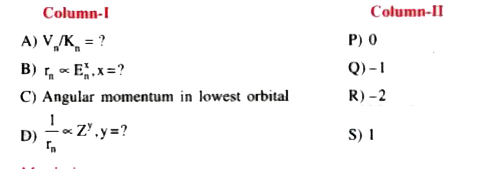
View SolutionUsing anhydrous AICI3 as catalyst which one of the following reactions produces ethyl benzene
View SolutionIn which of the following pairs, the two species are isostructural?
View SolutionIn Bohr's model of hydrogen atom ,
View SolutionThe Bohr model of atoms
View SolutionBohr atomic model explains
View SolutionDescribe Bohr model of the atom.
View SolutionAtomic model | Bohrs Model
View SolutionWrite down the postulates of Bohr atom model.
View SolutionAtoms|Bohr Model Of The Hydrogen Atom
View SolutionAccording to the Bohr model of atoms
View SolutionBOHR MODEL OF ATOM
View SolutionIntroduction|Bohr Model of Atom
View Solution
