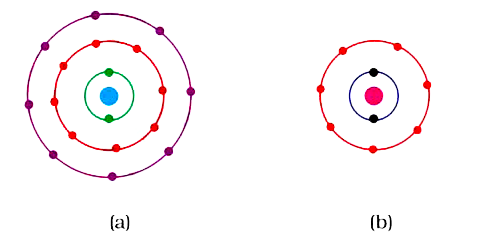
Find out the valency of the atoms represented by the Figure (a) and (b).

Video Solution
Text Solution
(a) It has electronic configuration
KLM288
Its outer shell complete octet.
Hence, its valency = 0
(b) It has electronic configuration
KL27
It can easily gain one electron to complete its outermost octet.
Hence, its valency = 1
|
Similar Questions
चित्र (a) और (b) द्वारा प्रदर्शित परमाणुओं की संयोजकता ज्ञात करें।
View SolutionValency of potassium atom : one : : Valency of argon atom : ________
View SolutionFind out the number of valence electrons and valency of the atoms represented in the following figures.
View SolutionFind out the number of valence electrons and valency of the atoms represented in the following figures.
View Solutionनिम्नलिखित में से कौन-सा परमाणु दो प्रकार की संयोजकता दर्शाता है?
View SolutionAn atom of an element contains 2. 8 and 5 electrons in K, L and M shell respectively. Find out :- valency of the element.
View SolutionA neutral atom of an element has 2K,8L and 5M electron .Find out the following
Number of electron in valence shell
View Solution
