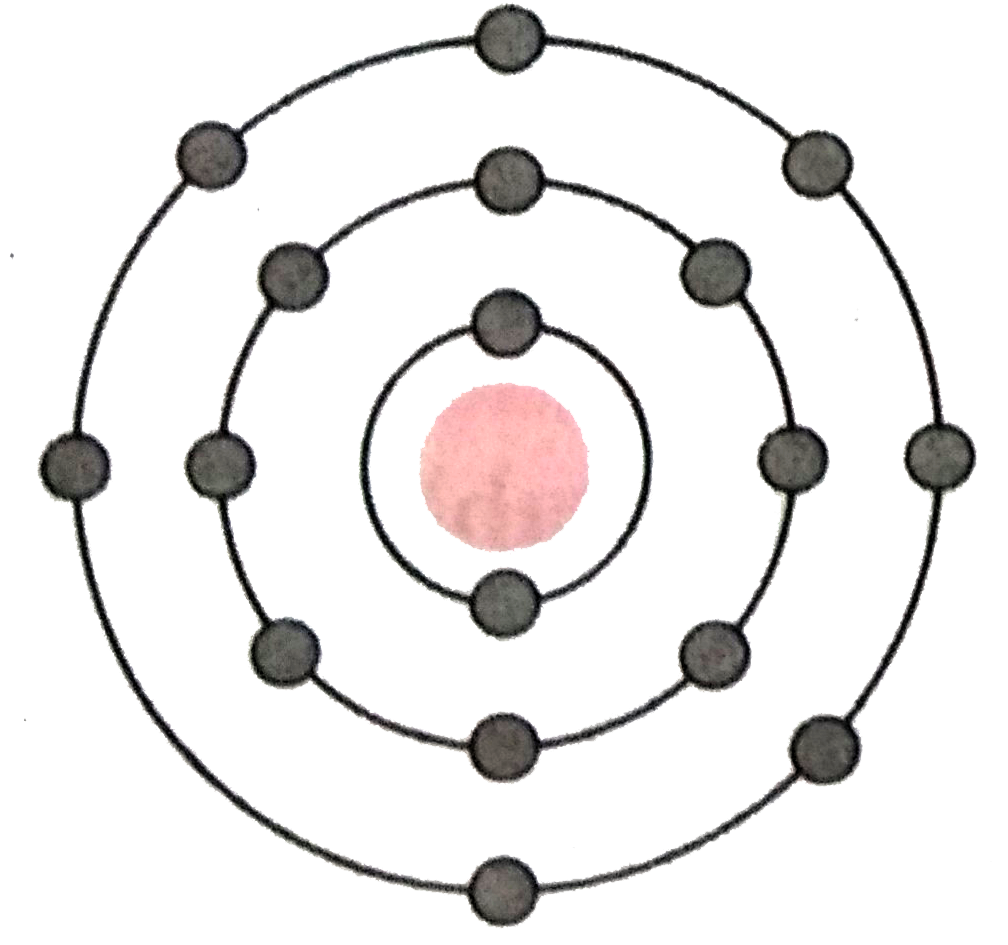
Find out the number of valence electrons and valency of the atoms represented in the following figures.
 Solution in Marathi
Solution in Marathi
Video Solution
Text Solution
The number of valence electrons is 7.
∴ Valency = 1
|
Similar Questions
Find out the number of valence electrons and valency of the atoms represented in the following figures.
View SolutionThe sum of number of valence electrons and valency of an element with atomic number 15 is_____.
View Solutionకార్బన్ పరమాణువు నందు గల వేలన్సీ ఎలక్ట్రానుల సంఖ్య _____
View SolutionFind out the valency of the atoms represented by the Figure (a) and (b).

View SolutionFind out the number of valence electrons and valency of the atoms represented in the following figures.
View SolutionA neutral atom of an element has 2K,8L and 5M electron .Find out the following
Number of electron in valence shell
View SolutionThe number of valence electrons in carbon atom is
View Solution
