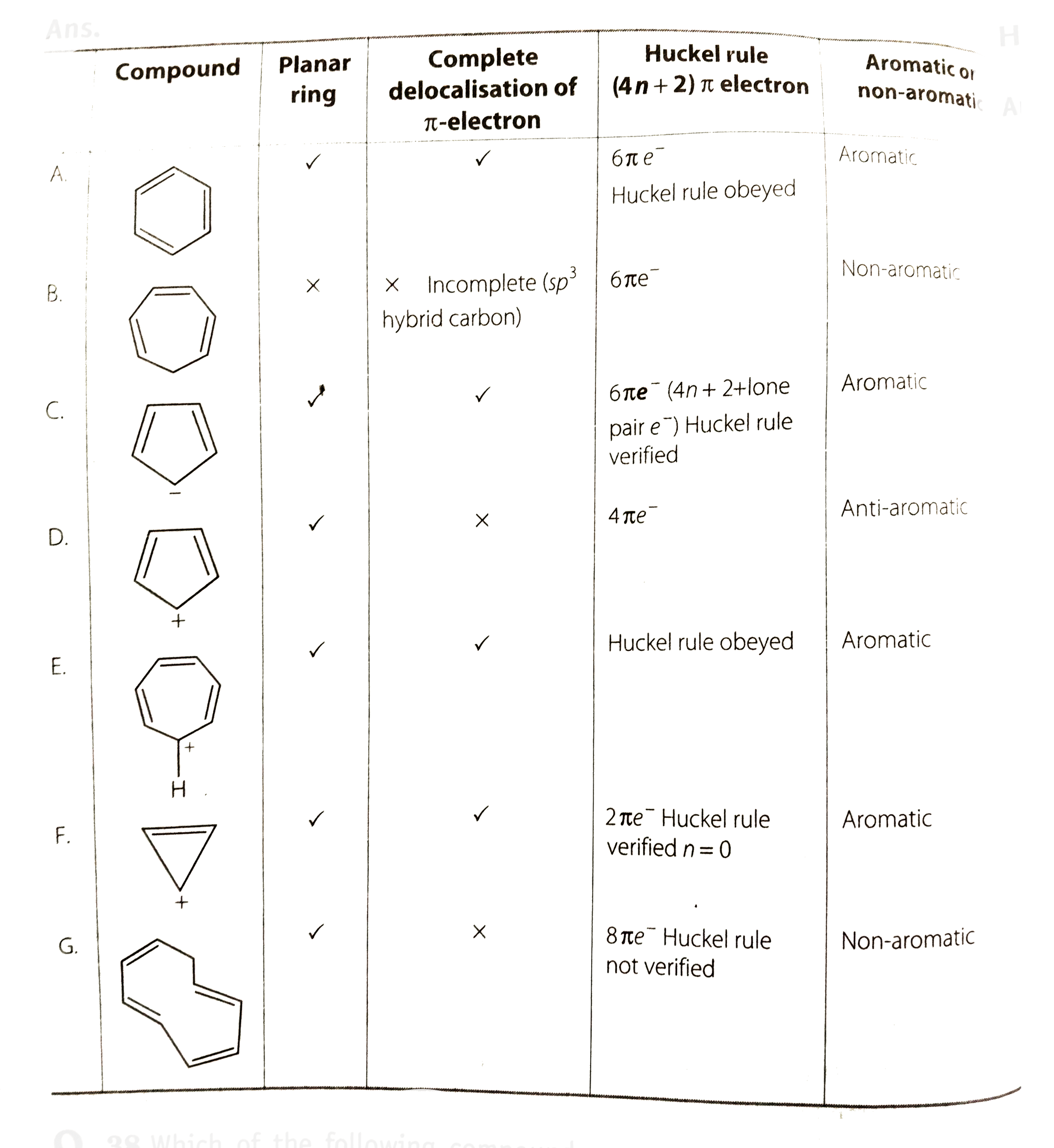
The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated π bonds .
(ii) complete dolocalisation of the π -electron in ring system i.e. , each atom in the ring has unbybridised p-orbital , and
(iii) Presence of (4n+2)π-electrons in the ring where n is an integer(n = 0, 1, 2, .......) [Huckel rule]. Using this information classify the following compounds as aromatic/non-aromatic.
![The ring systems having following characteristics are aromatic. (i) Planar ring containing conjugated pi bonds . (ii) complete dolocalisation of the pi -electron in ring system i.e. , each atom in the ring has unbybridised p-orbital , and (iii) Presence of (4n+2)pi-electrons in the ring where n is an integer(n = 0, 1, 2, .......) [Huckel rule]. Using this information classify the following compounds as aromatic/non-aromatic.](https://d10lpgp6xz60nq.cloudfront.net/physics_images/ARH_NCERT_EXE_CHM_XI_C13_S01_037_Q01.png)
Video Solution
Text Solution
|
Similar Questions
Knowledge Check
Which of the folloiwng pairs of compounds are ring - chain isomers?
A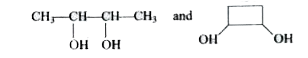 B
B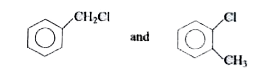 C
C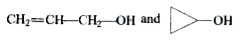 D
D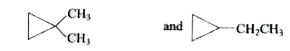
Which of the following ring compounds obeys Huckel's rule ?
AC4H−14BC4H+14CC4H−24DC4H4In which of the following molecules π−electron density in ring is maximum?
A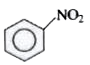 B
B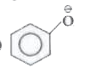 C
C D
D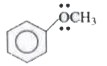
Signet ring stage is the characteristic of
View Solution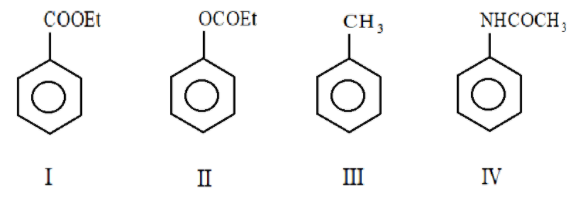
The correct order of electron density in aromatic ring of following compounds is:
View SolutionIn which of the following molecules π-electron density in ring is minimum :
View SolutionCambium ring in dicot system is
View SolutionCambium ring in dicot system is
View Solution