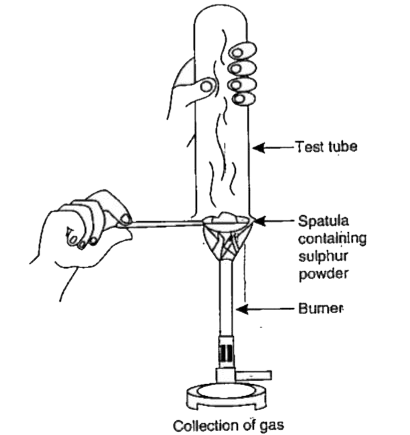
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in figure below:
(a) What will be the action of gas on (i) dry litmus paper ? (ii) moist litmus paper ?
(b ) Write a balanced chemical equation for the reaction taking place.
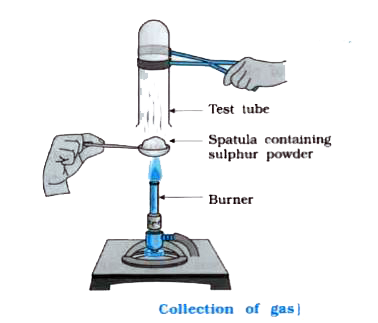
When sulphur powder is heated in air, it forms sulphur dioxide, which is acidic in nature and its aqueous solution is called sulphurous acid (H2SO3). (a) Action of gas : (i) There will be no effect of gas on dry litmus paper. (ii) Moist blue litmus paper changes its colour to red due to H+ ions present in the aqueous solution of H2SO3 formed from SO2 obtained after burning sulphur. (b) Balanced chemical equation for the above activity is as given below:
S(s)+O2(g)→SO2(g)
SO2(g)+H2O(l)H2SO3(aq)Sulphurous acid
Similar Questions
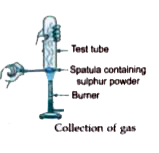
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in Fig. below : (a) What will be the action of gas on (i) dry litmus paper ? (ii) moist litmus paper ?
(b) Write a balanced chemical equation for the reaction taking place
View SolutionSameer took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in the figure.
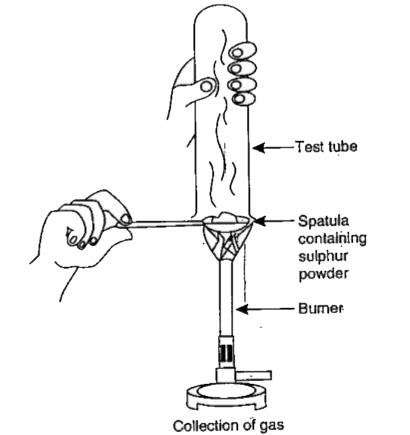
What will be the action of gas on dry litmus paper?
View SolutionSameer took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in the figure.
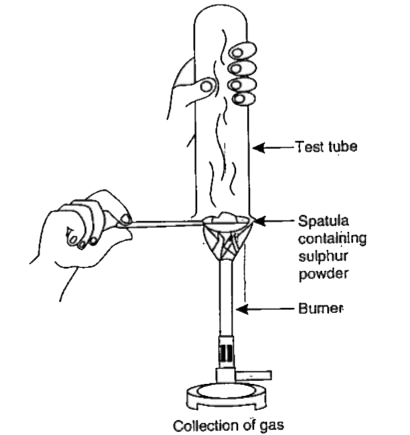
What will be the action of gas on moist litmus paper?
View SolutionPiyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over the burning sulphur.
(a) What will be the action of this gas on: (i)Dry litmus paper (ii) Moist litmus paper
View SolutionPratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.

(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
View SolutionSameer took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in the figure.
Write a balanced chemical equation for the reaction taking place.
View SolutionPiyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over the burning sulphur.
(b) Write a balanced chemical equation for the reaction taking place.
View SolutionMeetu heated sulphur powder in a spatula and collected the gas evolved.
(i) Give the equation of the reaction for heating of sulphur.
(ii) Write the effect of gas on red litmus solution and blue litmus solution.
(iii) Give the type of ore whose heating will evolve the gas.
View SolutionMeetu heated sulphur powder in a spatula and collected the gas evolved.
(i) Give the equation of the reaction for heating of sulphur.
(ii) Write the effect of gas on red litmus solution and blue litmus solution.
(iii) Give the type of ore whose heating will evolve the gas.
View SolutionPrateek took a piece of burning charcoal and collected the gas evolved in a test tube.
(a) How will he find the nature of the gas?
(b) Write down word equations of all the reactions taking place in this process?
View Solution
