State the pair of isobar elements.
4020Ca, 3517Cl
2018Ca, 2019Cl
168Ca, 188Cl
197Ca, 137Cl
The correct Answer is:A, B, C
Topper's Solved these Questions
- STRUCTURE OF THE ATOM BOOK - KUMAR PRAKASHANCHAPTER - STRUCTURE OF THE ATOM EXERCISE - ACTIVITY
2 Videos
- STRUCTURE OF THE ATOM BOOK - KUMAR PRAKASHANCHAPTER - STRUCTURE OF THE ATOM EXERCISE - ACTIVITY
2 Videos
- QUESTION PAPER 03BOOK - KUMAR PRAKASHANCHAPTER - QUESTION PAPER 03EXERCISE - SECTION -D
4 Videos
Similar Questions
Knowledge Check
Pair of isobar is :
A.776C,137NB.136C,147NC.146C,158NDNone of theseAn ionic compound will be formed by the combination of one of the following pairs of elements. This pair of element is :
Achlorine and calciumBcalcium and sodiumCsulphur and carbonDchlorine and chlorineWhich of these is a pairs of isobar
A6C12,8O16B6C13,6O14C20Ca40,18Ar10D20Ca40,18Ar10
Out of the elements H(1), Be(4), Na (11), Mg(12) :
(a) Write the pair of elements having similar chemical properties.
(b) State the group number of each pair.
(c) Name one another element belonging to each of these groups.
View SolutionWhat are isotopes ? Discuss the isotopes of any two elements . How do isotopes and isobars differ ?
View SolutionElements from A to F have in them the distribution of electrons, neutrons and protons as follows:
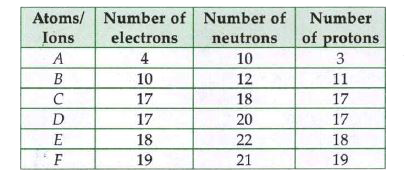
Making use of these data, find
a pair of isobars
View SolutionFind the number of isobaric pairs for the elements repesented by (A,z)
(232,90),(228,88),(228,89),(214,82),(218,84),(210,81),(216,85),(207,82),(211,83):
View SolutionThe atoms of which of the following pair of elements are most likely to exist in free state ?
View Solution
