If we plot volume of a certain mass of a gas against temperature at constant pressure, we get a straight line intersecting on the negative side at −273.∘C which explains about absolute zero . This graph is known as
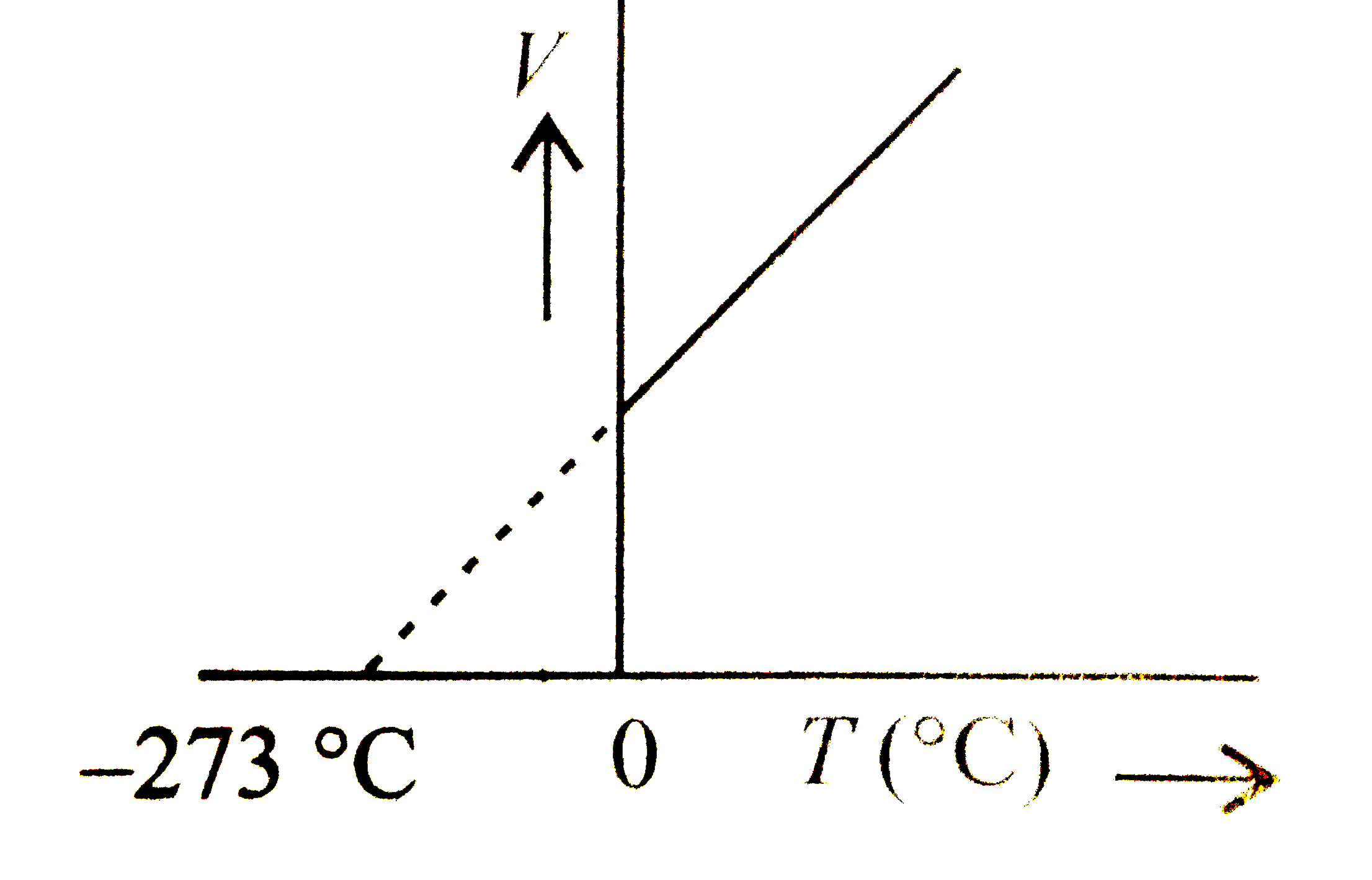
isochor
isotherm
isotone
isobar
The correct Answer is:D
Step by step video & image solution for If we plot volume of a certain mass of a gas against temperature at constant pressure, we get a straight line intersecting on the negative side at - 273 .^(@)C which explains about absolute zero . This graph is known as by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
At 1 atmospheric pressure and 0.∘C , certain mass of a gas measures 0.4L . Keeping the pressure constant , if the temperature is increased is increased to 273.∘C , what will be its volume ?
A0.8 LB22.4 LC54.6 LD0.4 L
The temperature of a given mass of a gas is increased from 19∘C to 20∘C at constant pressure. The volume V of the gas is
View SolutionWhich of the following curves obtained at constant temperature and for a given mass of a gas is not a straight line ?
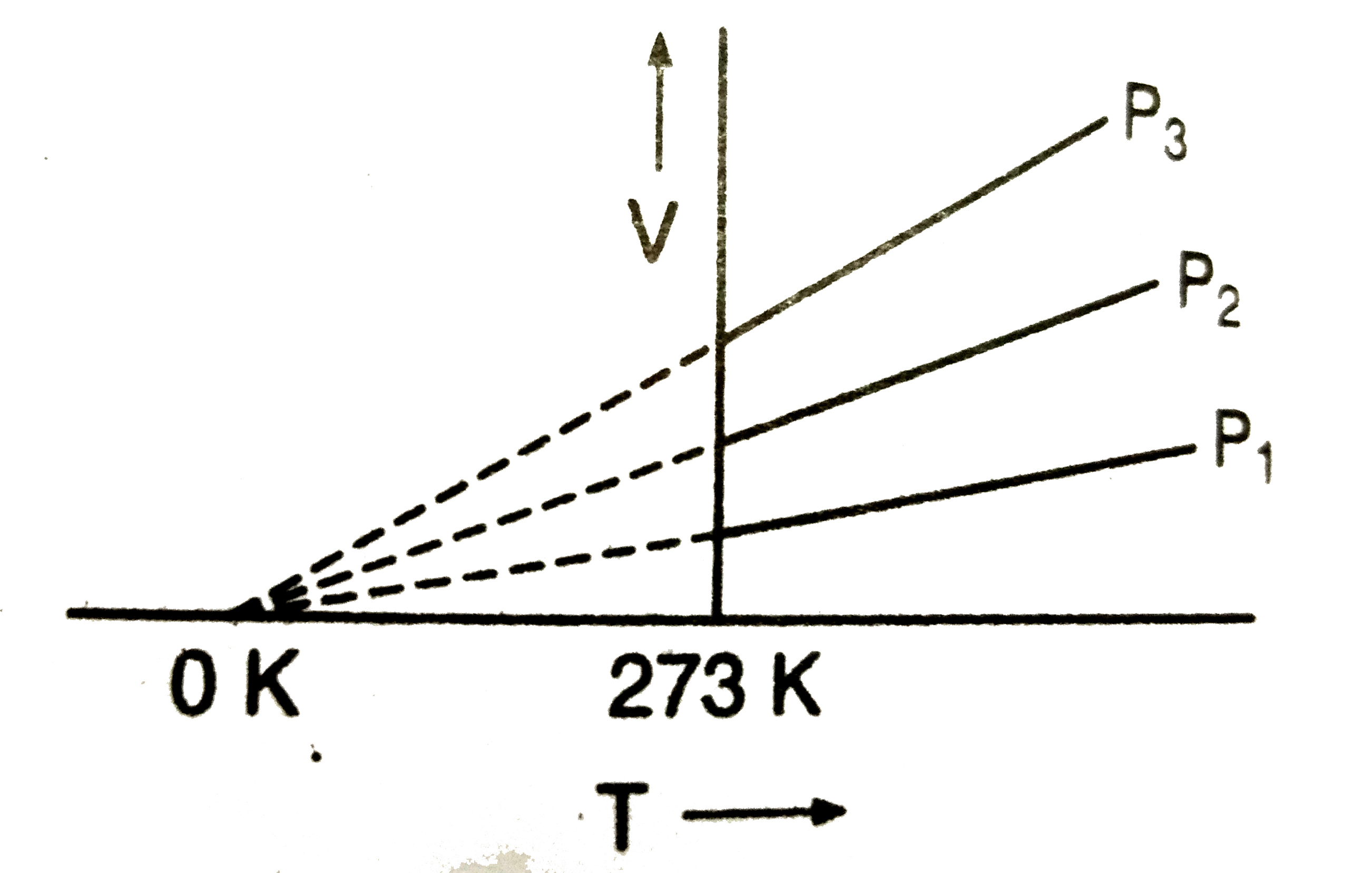
View SolutionThe volume-temperature graphs of a given mass of an ideal gas at constant pressure are shown below. What is the correct order of pressure ?
View SolutionThe volume of a given mass of a gas is 100ml at 100°C. If pressure is kept constant at what temperature will the sample have the volume of 200ml?
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in figure. Which of the following order of pressure is correct for this gas ?
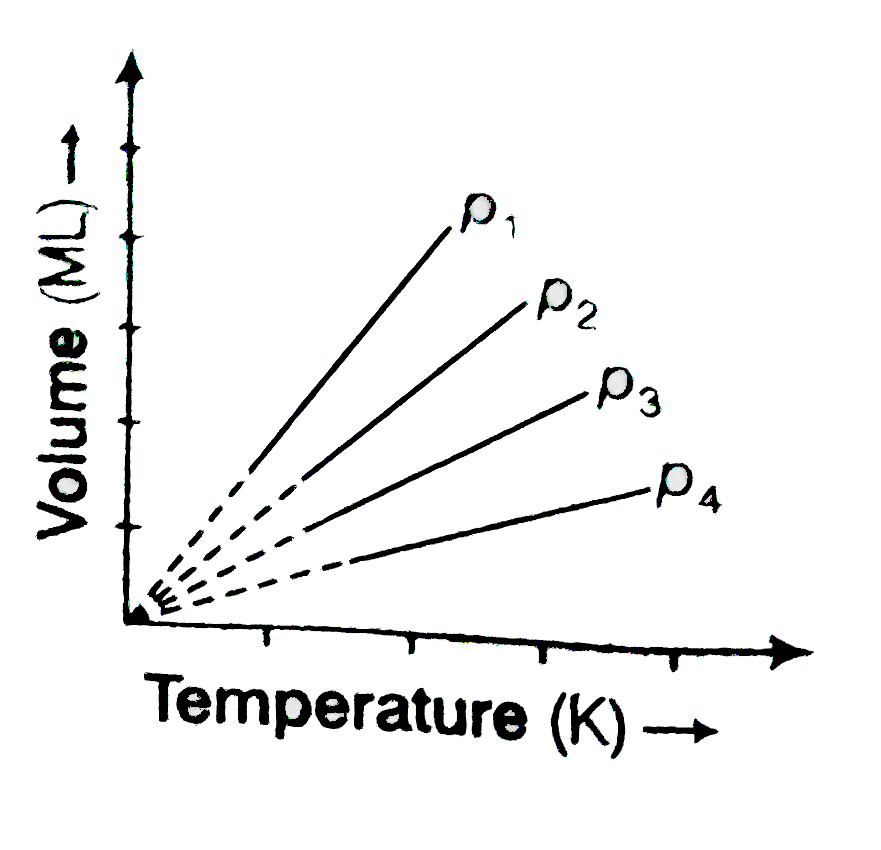
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin . The plots at different values of pressure are shown in figure .
Which of the following order of pressure is correct for this gas ?
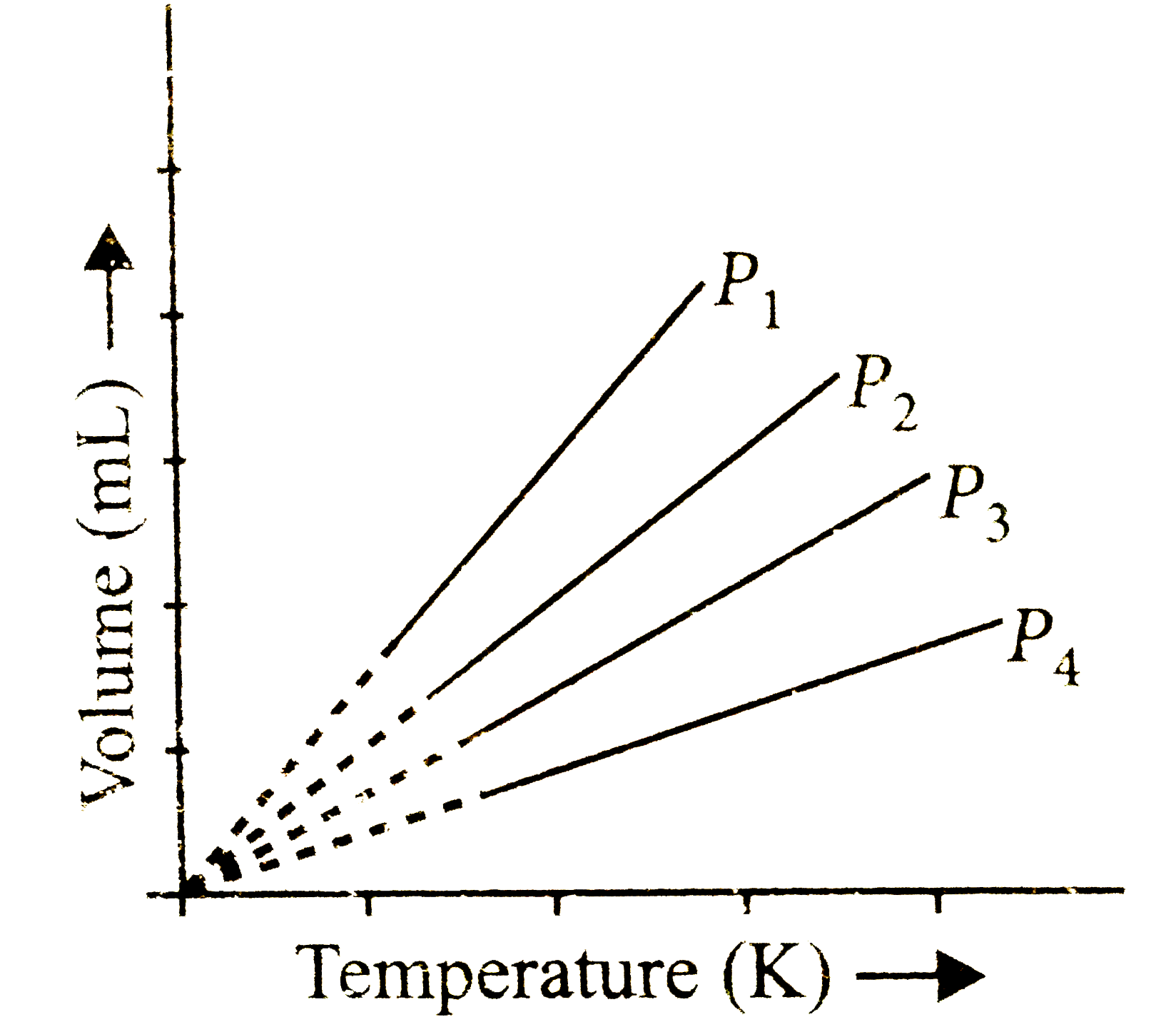
View SolutionA plot of volume (V) versus temperature (T) for a gas at constannt pressure is a straight line passing through the origin. The plots at different values of pressure are shown in figure. Which of the following order of pressure is correct for this gas?
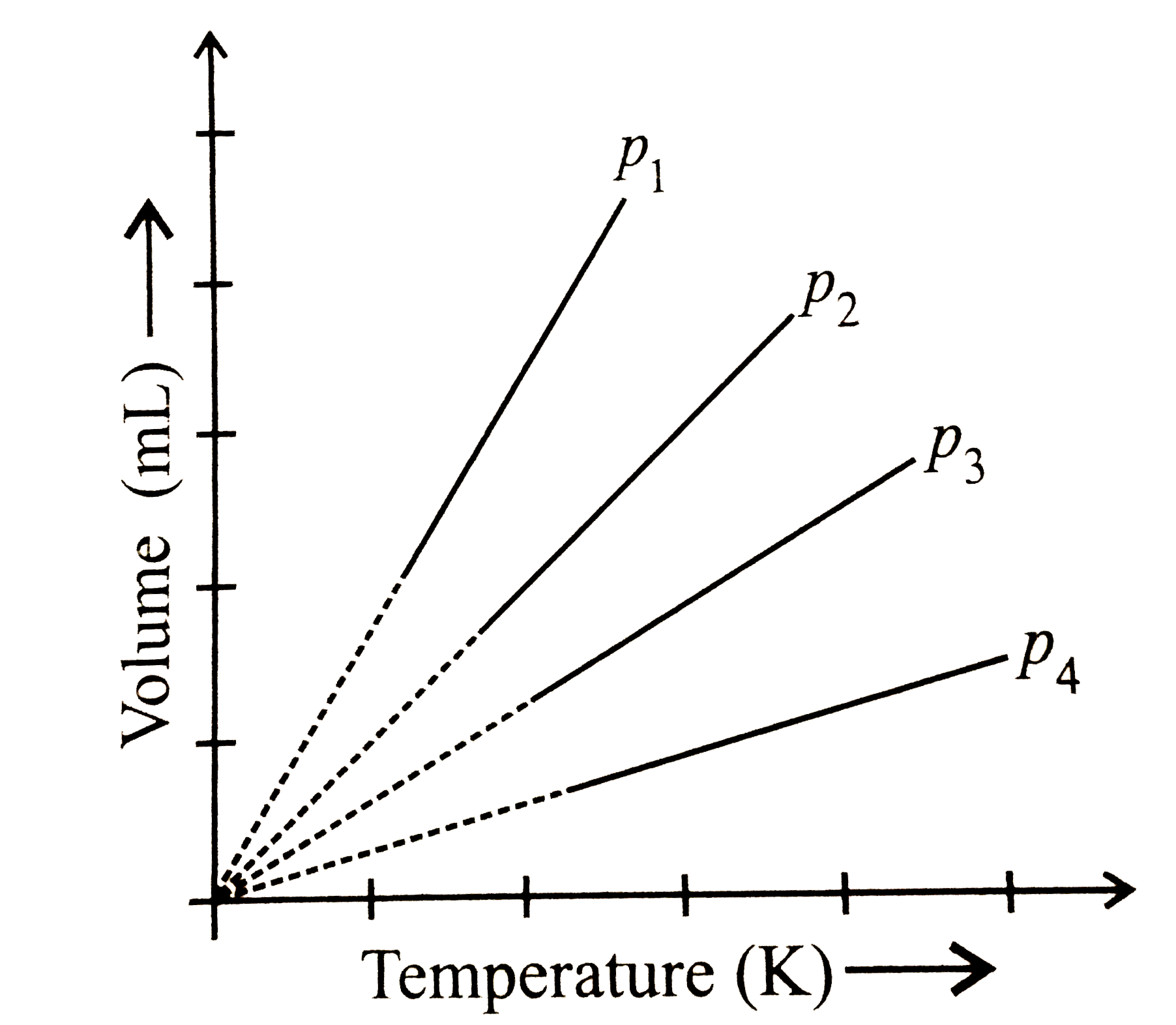
View Solution
