Which of the following will have maximum dipole moment?
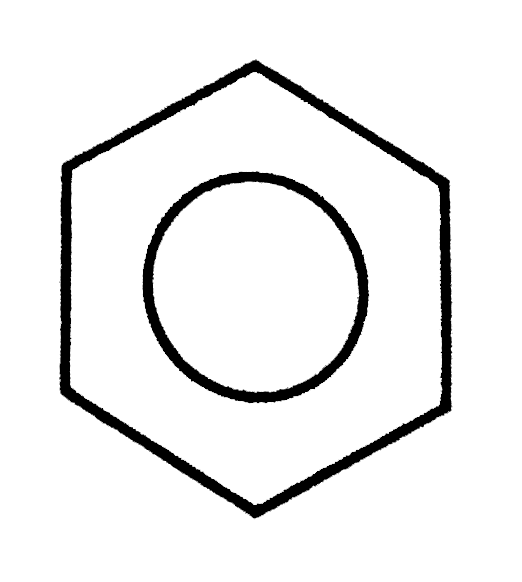
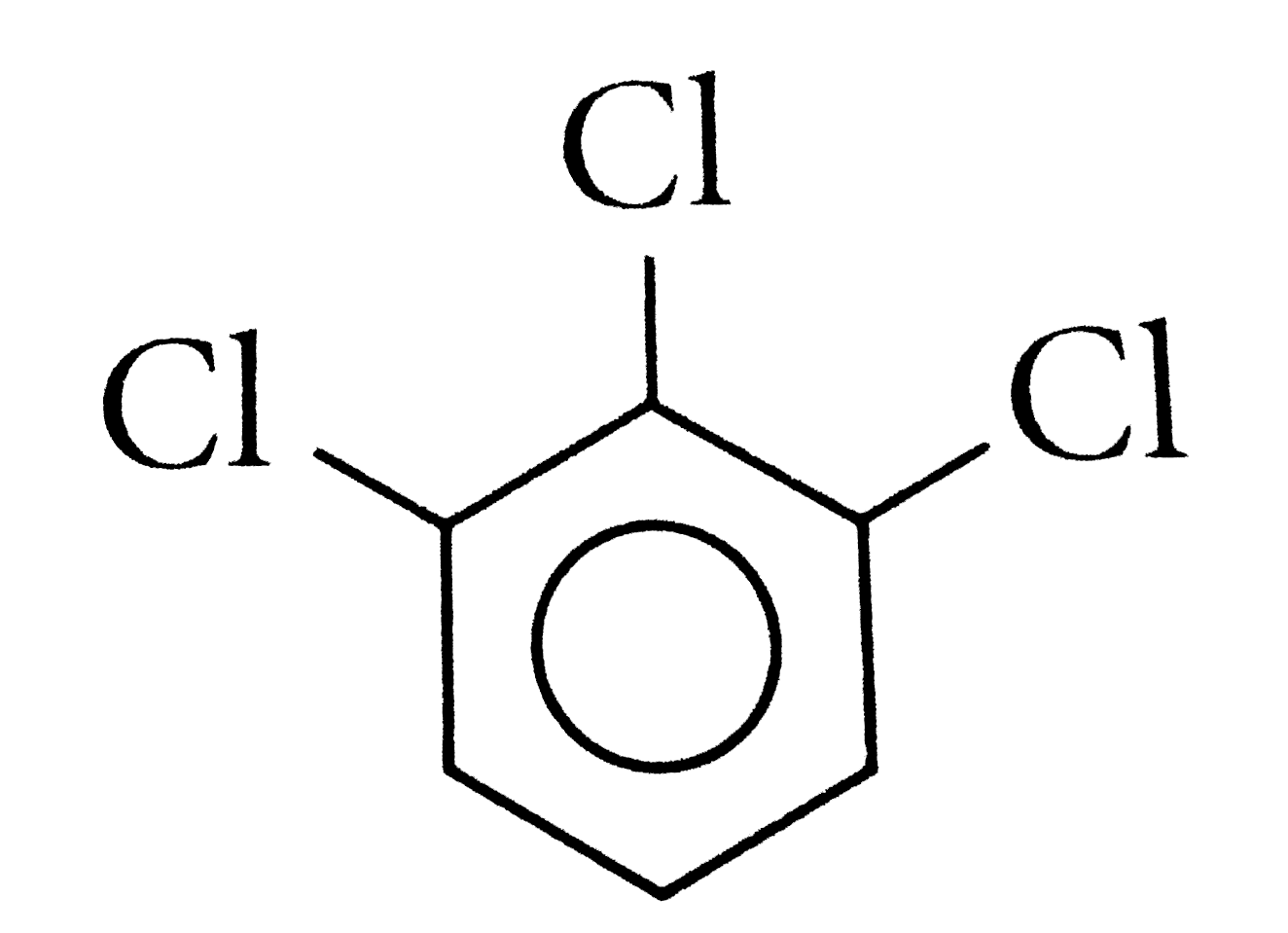
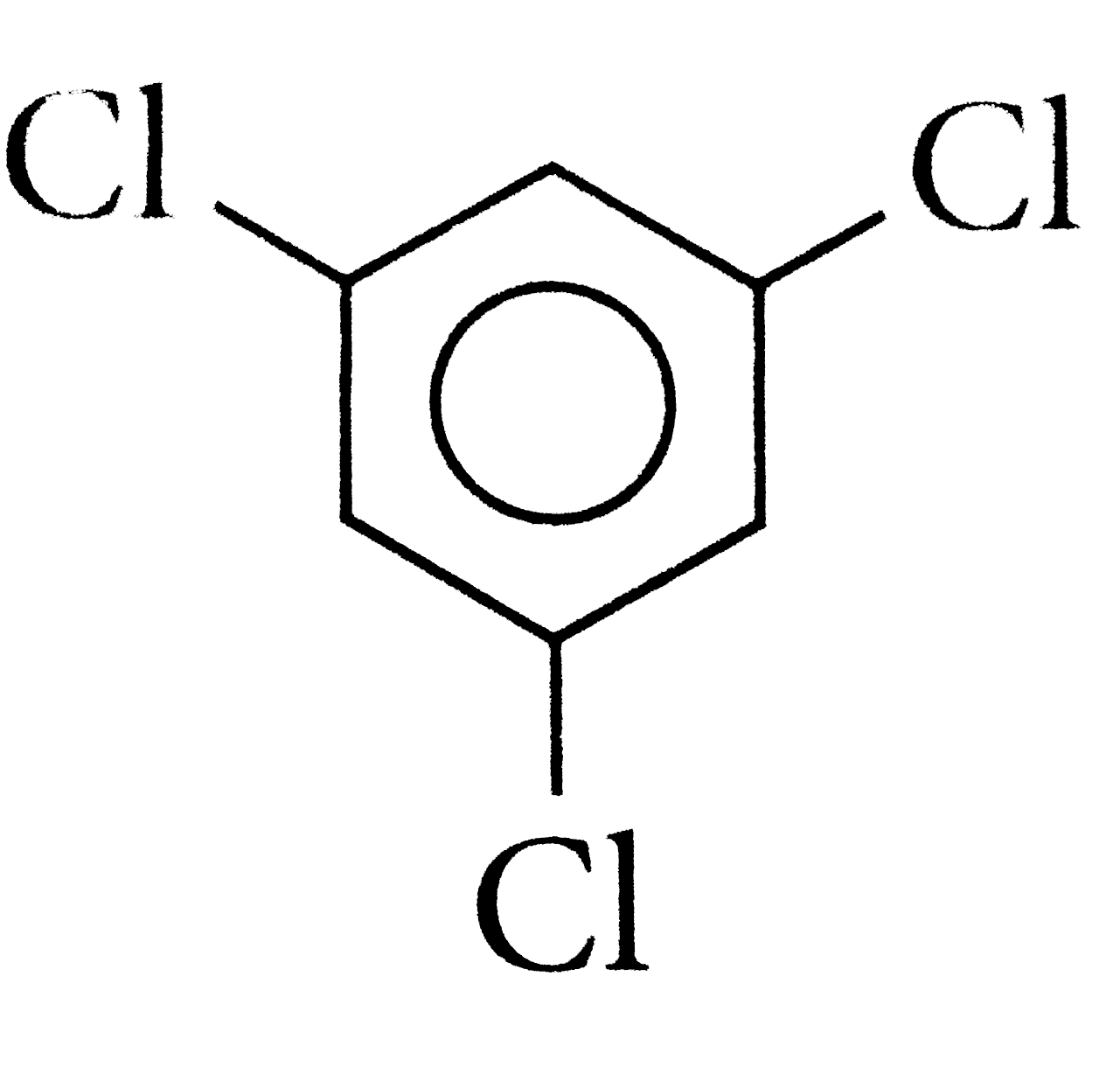
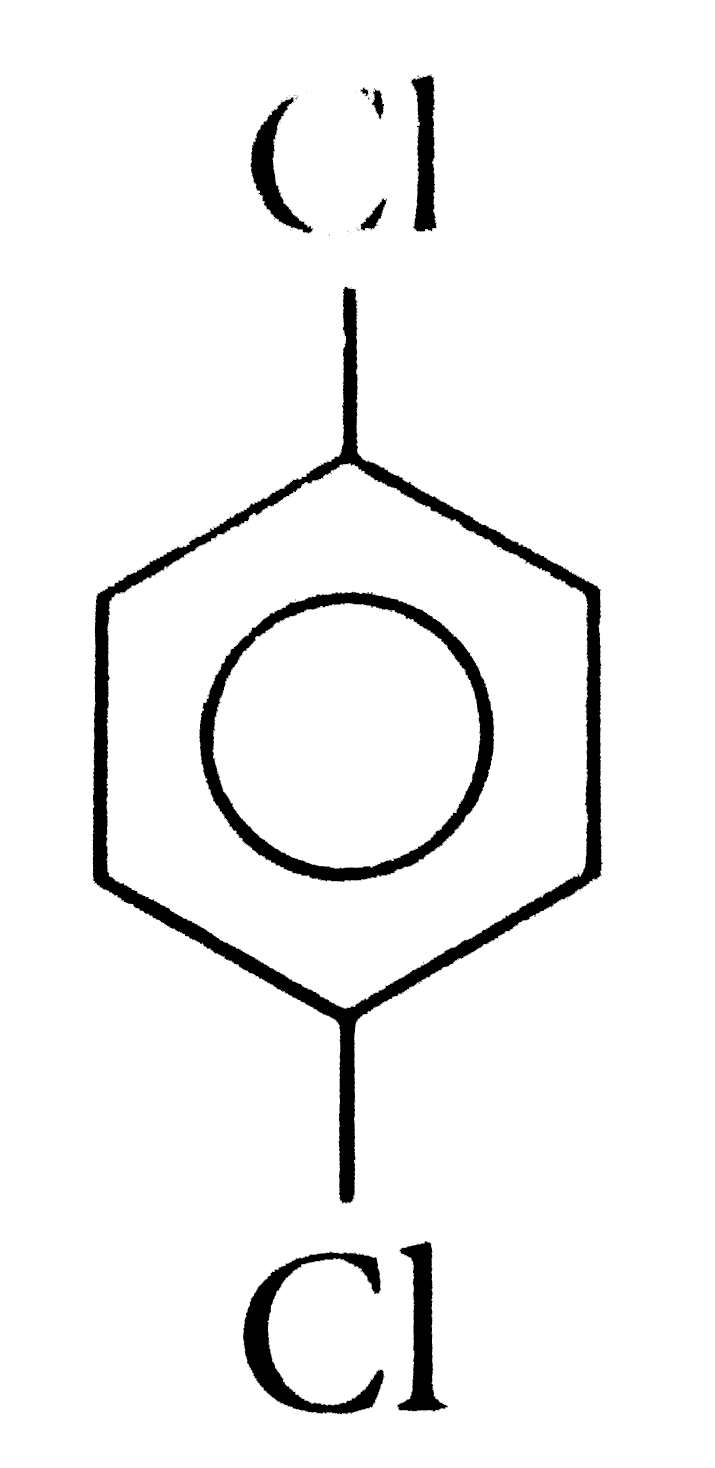
The correct Answer is:B
To determine which of the given options has the maximum dipole moment, we need to analyze the molecular structure and the electronegativity of the atoms involved in each option. Here’s a step-by-step solution:
Step 1: Understand Dipole Moment
The dipole moment (μ) is a measure of the separation of positive and negative charges in a molecule. It is calculated as:
μ=Q×r
where Q is the magnitude of the charge and r is the distance between the charges. A higher dipole moment indicates a greater polarity in the molecule.
Step 2: Analyze Each Option
Assuming the options are A, B, C, and D, we will analyze them based on their molecular structures and electronegativity differences.
1. Option A: This could be a non-polar molecule like benzene (C6H6). Since all carbon atoms have the same electronegativity, there is no charge separation, resulting in a dipole moment of zero.
2. Option B: This could be a polar molecule, such as chloromethane (CH3Cl). The electronegativity difference between carbon and chlorine creates a dipole moment directed towards chlorine, resulting in a non-zero dipole moment.
3. Option C: This could be another non-polar molecule, such as carbon dioxide (CO2). Although it has polar bonds, the linear shape of the molecule causes the dipoles to cancel each other out, resulting in a net dipole moment of zero.
4. Option D: This could be a symmetrical molecule like tetrafluoromethane (CF4). Similar to CO2, the symmetrical shape leads to cancellation of dipoles, resulting in a dipole moment of zero.
Step 3: Compare Dipole Moments
From the analysis:
- Options A, C, and D are non-polar and have a dipole moment of zero.
- Option B is polar and has a non-zero dipole moment due to the electronegativity difference between carbon and chlorine.
Conclusion
Thus, the option with the maximum dipole moment is Option B.
The dipole moment (μ) is a measure of the separation of positive and negative charges in a molecule. It is calculated as:
μ=Q×r
where Q is the magnitude of the charge and r is the distance between the charges. A higher dipole moment indicates a greater polarity in the molecule.
Assuming the options are A, B, C, and D, we will analyze them based on their molecular structures and electronegativity differences.
2. Option B: This could be a polar molecule, such as chloromethane (CH3Cl). The electronegativity difference between carbon and chlorine creates a dipole moment directed towards chlorine, resulting in a non-zero dipole moment.
From the analysis:
- Options A, C, and D are non-polar and have a dipole moment of zero.
- Option B is polar and has a non-zero dipole moment due to the electronegativity difference between carbon and chlorine.
Thus, the option with the maximum dipole moment is Option B.
Similar Questions
Knowledge Check
Which has maximum dipole moment ?
A B
B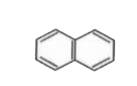 C
C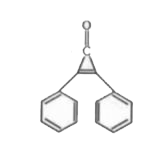 D
D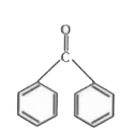
Which of the following compounds have a dipole moment?
A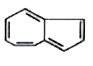 B
B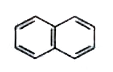 C
C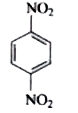 D
D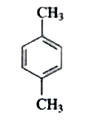
Match the following:
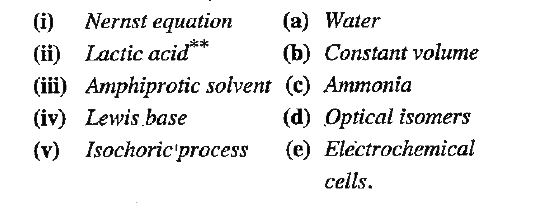 AA and DBB and CCC and EDB and E
AA and DBB and CCC and EDB and E

