α-hydroxypropanoic acid can be prepared from ethanal by following the steps given in the sequence.
Treat with HCN followed by acidic hydrolysis.
Treat with NaHSO3 followed by reaction with Na2CO3.
Treat with H2SO4 followed by hydrolysis.
Treat with K2Cr2O7 in presence of suphuric acid.
The correct Answer is:A
To prepare alpha-hydroxypropanoic acid from ethanal, we can follow these steps:
Step 1: React Ethanal with HCN
- Ethanal (acetaldehyde) is treated with hydrogen cyanide (HCN). This reaction leads to the formation of a cyanohydrin. The reaction can be represented as follows:
CH3CHO+HCN→CH3C(OH)(CN)H
Here, the cyanide ion adds to the carbonyl carbon of ethanal, resulting in the formation of 2-hydroxypropanenitrile (cyanohydrin).
Step 2: Hydrolysis of the Cyanohydrin
- The next step involves the hydrolysis of the cyanohydrin. This can be achieved by treating it with water in the presence of an acid (typically dilute sulfuric acid). The hydrolysis reaction can be represented as follows:
CH3C(OH)(CN)H+H2OH+−−→CH3C(OH)(COOH)H
In this step, the nitrile group (-CN) is converted into a carboxylic acid (-COOH), resulting in the formation of alpha-hydroxypropanoic acid (lactic acid).
Final Product
- The final product obtained from these reactions is alpha-hydroxypropanoic acid, which has the following structure:
CH3C(OH)(COOH)H
Conclusion
- Therefore, the overall process involves the addition of HCN to ethanal followed by hydrolysis to yield alpha-hydroxypropanoic acid.
---
- Ethanal (acetaldehyde) is treated with hydrogen cyanide (HCN). This reaction leads to the formation of a cyanohydrin. The reaction can be represented as follows:
- The next step involves the hydrolysis of the cyanohydrin. This can be achieved by treating it with water in the presence of an acid (typically dilute sulfuric acid). The hydrolysis reaction can be represented as follows:
- The final product obtained from these reactions is alpha-hydroxypropanoic acid, which has the following structure:
- Therefore, the overall process involves the addition of HCN to ethanal followed by hydrolysis to yield alpha-hydroxypropanoic acid.
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBOOK - NCERT FINGERTPS ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDSEXERCISE - MCQs( NOMENCLATURE AND STRUCTURE OF CARBOXYL GROUP )
2 Videos
- ALCOHOLS,PHENOLS AND ETHERSBOOK - NCERT FINGERTPS ENGLISHCHAPTER - ALCOHOLS,PHENOLS AND ETHERSEXERCISE - Assertion And Reason
14 Videos
- AMINES BOOK - NCERT FINGERTPS ENGLISHCHAPTER - AMINES EXERCISE - ASSERTION & REASON CORNER
14 Videos
Similar Questions
Knowledge Check
Propanone can be prepared from ethyne by
Apassing a mixture of ethyne and steam over a catalyst, magnesium at 420∘CBpassing a mixture of ethyne and ethanol over a catalyst zinc chromiteCboiling ethyne with water and H2SO4Dtreating ethyne with lodine and NaOH.Acetylene can be prepared from
Apotassium fumerateBcalcium carbideCethylene bromideDall of these.Acid anhydride can be prepared by :
AC6H5COOH+CH3COClpyridine−−−−→BC6H5COO−Na++C6H5COCl→CC6H5CONH2+CH3COO−Na+→D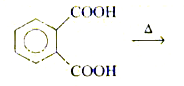
Bacteria can prepare food from
View SolutionHydrogen from HCl can be prepared by
View Solution2-Butyne can be prepared from which of following compounds?
View SolutionAlcohol can be prepared from alken by which of the following reaction
View SolutionThe given imine can be prepared from which of the following reactions?
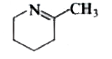
View Solution

