Two moles of helium gas undergo cyclic processes shown as assuming the gas to be ideal.calculate the following quantities in this process. (a) The net change in the heat energy (b) The network done (C) The net change in internal energy. (TA=300K,TB=400K)
We shall first calculate here work done in the cyclic process. As AB is isobaric process, hence work done during this process from A to B is
WAB=PA(VB−VA)=PBVB−PAVA(∴PA=PB)
According to perfect gas equation, PAVA=nRTBandPBVB=nRTB
∴WAB=μRT(TB−TA)2×8.32×(400−300=2×832=1664 joule
work done during isothermal process from B to C
WBC=nRTloge(VfVi)=μRTCloge(VCVB)=μRTClog(PBPC)[∴PBVB=PCVC]
=2×8.32×400×loge21[∴PB=2andPC=1atm]=4068 joule
Work done during isobaric process from C to D
WCD=nR(TD−TC)2×8.32×(300−400)=−1664 joule
Work done during isothrmal process from D to A
WDA=nRTloge(VfVi)=nRTDloge(VAVD)=nRTDloge(Pd/PA)[∴PAVA=PDVD]
=−2×8.32×300×loge2=−3456 joule
∴ Net work done =WAB+WBC+WCD+WDA=1664+4608−1664−3456=1152 joule
(a) In a cyclic process ΔU=0∴ From first law of thermodynamics
Net change in heat energy ΔQ=Δw=1152 joule
(b ) Net work done ΔW=11.52 joule
(c) Net change in internal energy ΔU=0.
Topper's Solved these Questions
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:TEST YOUR SELF
Explore 17 Videos
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:SHORT ANSWER TYPE QUESTIONS
Explore 8 Videos
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ((ADVANCED) INTEGER/SUBJECTIVE TYPE QUESTIONS))
Explore 11 Videos
- THERMAL PROPERTIES OF MATTERBook:AAKASH SERIESChapter:THERMAL PROPERTIES OF MATTERExercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions
Explore 2 Videos
- THERMOMETRY Book:AAKASH SERIESChapter:THERMOMETRY Exercise:Numerical Exercise (LEVEL- 1)
Explore 11 Videos
Similar Questions
Two moles of helium gas undergo a cyclic process as shown in Fig. Assuming the gas to be ideal, calculate the following quantities in this process
(a) The net change in the heat energy
(b) The net work done
(c) The net change in internal energy
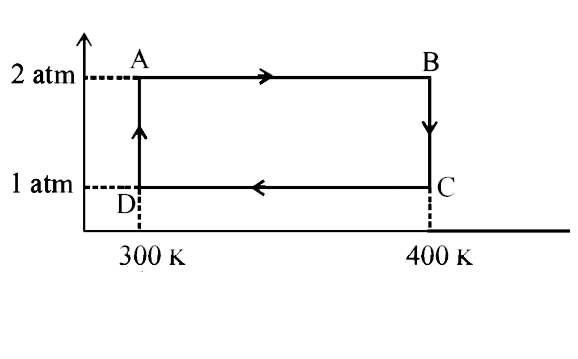
View SolutionTwo moles of helium gas undergo a cyclic process as shown in Fig. Assuming the gas to be ideal, calculate the following quantities in this process

(a) The net change in the heat energy
(b) The net work done
(c) The net change in internal energy
View Solution
Knowledge Check
Two moles of helium gas undergo a cyclic process as shown in figure. Assuming the gas to be ideal. The net work done by the gas is
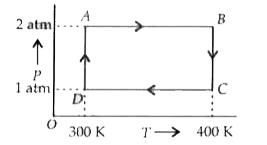 A200 Rln2B100 Rln2C300 Rln2D400 Rln2
A200 Rln2B100 Rln2C300 Rln2D400 Rln2Two moles of helium gas undergo a cyclic process as shown in figure. Assuming gas to be ideal, the net heat exchange in the process will be
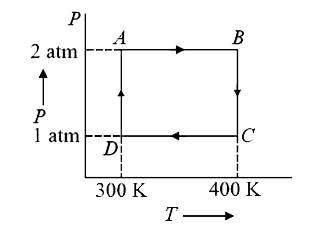 A1000 JB1152 JC1200 JD1500 J
A1000 JB1152 JC1200 JD1500 JTwo moles of Helium gas undergo a reversible cyclic process as shown in figure. Assuming gas to be ideal, what is the net work involved in the cyclic process ?
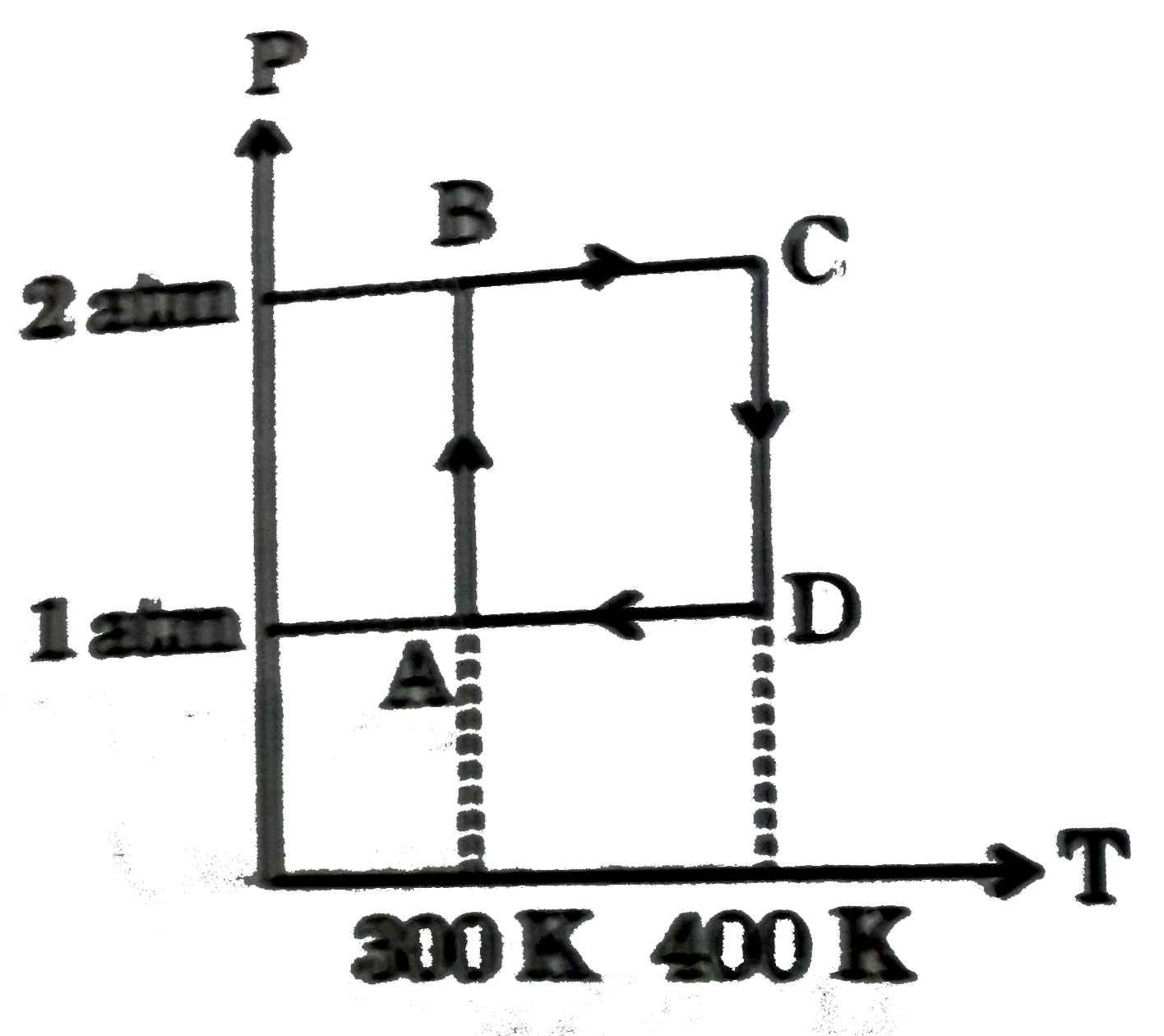 A−100Rln4B+100Rln4C+200Rln4D−200Rln4
A−100Rln4B+100Rln4C+200Rln4D−200Rln4
Two moles of helium gas undergo a cyclic process as shown in Fig. Assuming the gas to be ideal, calculate the following quantities in this process

(a) The net change in the heat energy
(b) The net work done
(c) The net change in internal energy
View SolutionTwo moles of helium gas undergo a cyclic process as shown in Fig. Assuming the gas to be ideal, calculate the following quantities in this process

(a) The net change in the heat energy
(b) The net work done
(c) The net change in internal energy
View SolutionTwo moles of helium gas undergo a cyclic process as shown in Fig. 19.2.14. Assuming the gas to be ideal, calculate the following quantitites in this process
(a) the net change in heat energy
(b) the net work done
(c) the net change in internal energy.
View SolutionTwo moles of Helium gas undergo a reversible cyclic process as shown in figure. Assuming gas to be ideal, what is the net work involved in the cyclic process ?

View SolutionTwo moles of Helium gas undergo a reversible cyclic process as shown in figure. Assuming gas to be ideal, what is the net work involved in the cyclic process ?

View Solution

