In which of the following reaction, hybridisation of carbon atom containing functional group does not change
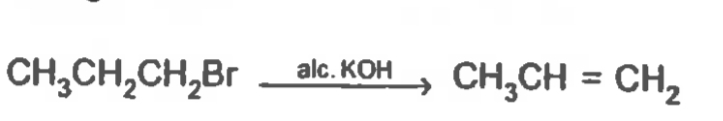
CH3CH2BrAlcohol, KOH−−−−−−−→
CH3−CH2−CH2OHAl2O3Δ−−−−→
CH3−CH=CH2+Cl2→
CH3−CH2I+KOH→
The correct Answer is:D
(A) CH3↑⏐
⏐⏐sp3CH2↑⏐
⏐⏐sp3BrAlcohol−−−→KOHCH2↑⏐
⏐⏐sp2=CH2↑⏐
⏐⏐sp2=CH2↑⏐
⏐⏐sp2+KBr+H2O
(B) CH3↑⏐
⏐⏐sp3−CH2↑⏐
⏐⏐sp3−CH2↑⏐
⏐⏐sp3OHAl2O3−−−→ΔCH3↑⏐
⏐⏐sp3−CH↑⏐
⏐⏐sp2=CH2↑⏐
⏐⏐sp2+H2O
(C )CH3↑⏐
⏐⏐sp3−CH2↑⏐
⏐⏐sp2=CH2↑⏐
⏐⏐sp2+Cl2→CH3↑⏐
⏐⏐sp3−CHCl↑⏐
⏐⏐sp3−CH2Cl↑⏐
⏐⏐sp3
(D) CH3↑⏐
⏐⏐sp3−CH2↑⏐
⏐⏐sp3−I+KOH→CH3↑⏐
⏐⏐sp3−CH2↑⏐
⏐⏐sp3−OH+KI
Similar Questions
Hybridisation of carbon atom in the functional group of an organic carboxylic acid is
View SolutionWhich of the following compounds contain all the carbon atoms in the same hybridisation state ?
View SolutionWhich of the following compounds contain after the carbon atoms in the same hybridisation state?
View SolutionIn the following elimination reaction, hybridisation of carbon atom to which halogen is attached changes from
View SolutionIn which of the following reaction, hybridisation of carbon atom containing functional group does not change
View SolutionWhich of the following compounds contain after the carbon atoms in the same hybridisation state?
View SolutionIn which of the following combination hybridisation of central atom (**) does not change ?
View Solution

