Calculate the packing efficiency in a simple cubic lattice. Solution in Kannada
a) Packing efficiency is a percentage of total space filled by the particles in a crystal.
The unit cell of a simple cube is
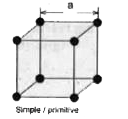
A simple cubic unit cell consists of only one atom/ particle/ sphere per unit cell. Let .a. be the edge length of the unit cell and .r. be the radius of the sphere.
We know that volume of the sphere =43πr3
The spheres/ lattice points at the corners in the simple cubic unit cell are touching each other. Therefore edge length =a=2r
Similarly volume of the cube =a3=2r3=8r3
Percentage of the space occupied by spheres
=Volume of the sphereVolume of the cube×100=43πr38r3×100
=4×100×228×3×7
=52.38%≈52.4% is the packing fraction in simple cubic lattice
Similar Questions
Write the steps involved to calculate the percentage of efficiency of packing of simple cubic crystal Or packing efficiency for simple cubic unit cell? Or Calculate the paking efficiency of metal crystal that has simple cubic structure?
View SolutionPacking Efficiency|Packing Efficiency In Simple Cubic Lattice|Packing Efficiency In CCP Structures|Summary
View SolutionPacking efficiency is the percentage of total space filled by the particles.
Which of the following lattices has the highest packing efficiency?
Simple cubic lattice, body centered cubic lattice, hexagonal close packed lattice
View SolutionCalculate the packing efficiency in simple cubic unit cell.
View SolutionShow that the packing efficiency of simple cubic lattice 47.6%.
View SolutionThe packing efficiency in case of a simple cubic lattice is.
View SolutionWhich of the following lattices has the highest packing efficiency?simple cubic or closed packed (ccp) lattice
View SolutionCalculate the packing efficiency of a metal crystal for a simple cubic lattice.
View Solution
