1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V2=4V1 then the ratio of temperatures T1T2 will be
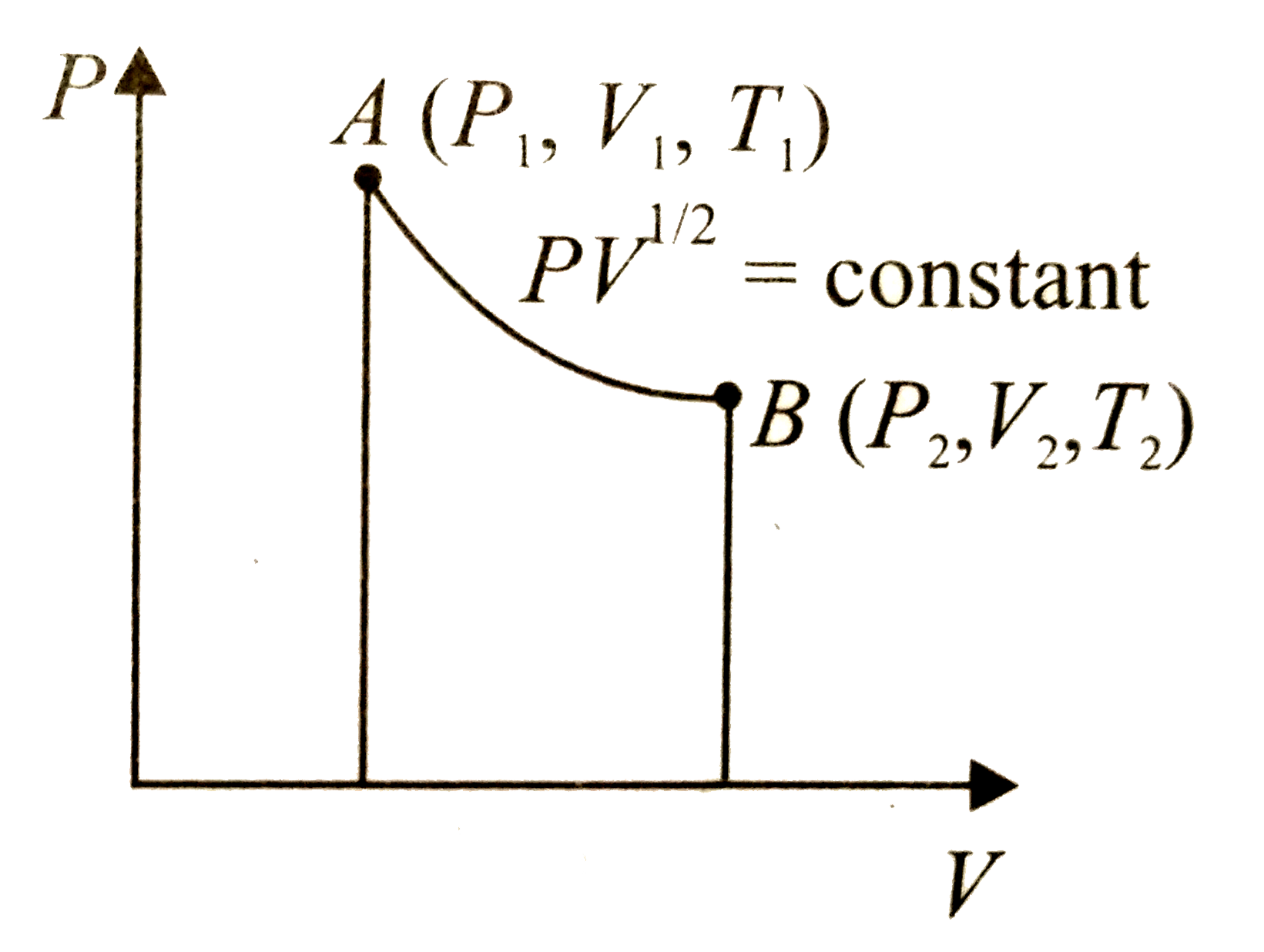
12
2
√2
1√2
The correct Answer is:C
Step by step video & image solution for 1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V_(2)=4V_(1) then the ratio of temperatures (T_(1))/(T_(2)) will be by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
The coefficient of static friction between a wooden block of mass 0.5 kg and a vertical rough wall is 0.2. The magnitude of horizontal force that should be applied on the block to keep it adhere to the wall will be ____N.
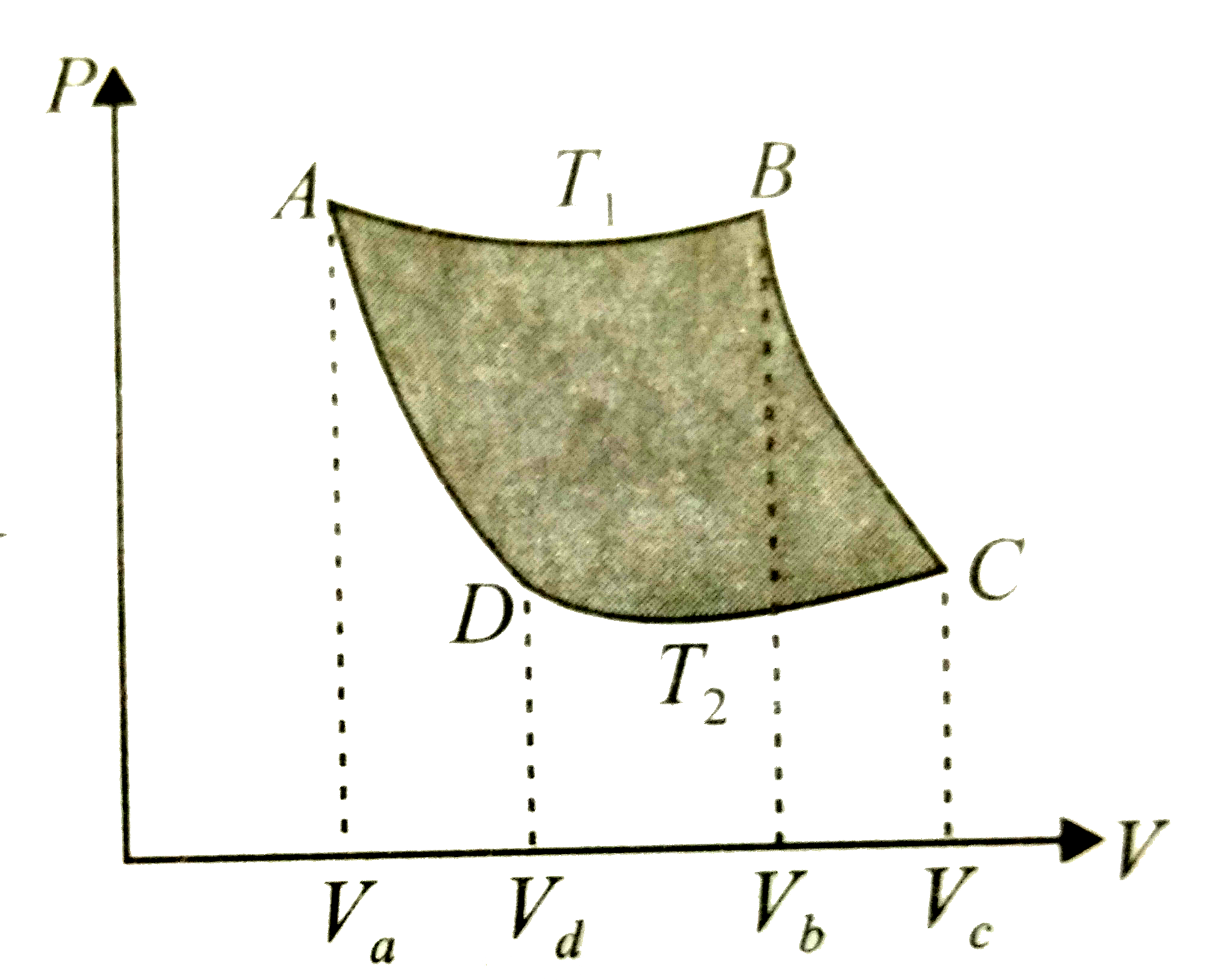
View SolutionIn the following P-V diagram two adiabatics cut two isothermals at temperature T_(1) and T_(2) (fig). The value of V_(a)/V_(d) will be
View SolutionConsider a P−V diagram in which the path followed by one mole of perfect gas in a cyclinderical container is shown in (figure)
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperatures T1/T2,ifV2=2V1?
(c ) Given the internal energy for one mole of gas at temperature Tis(3/2)RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1.
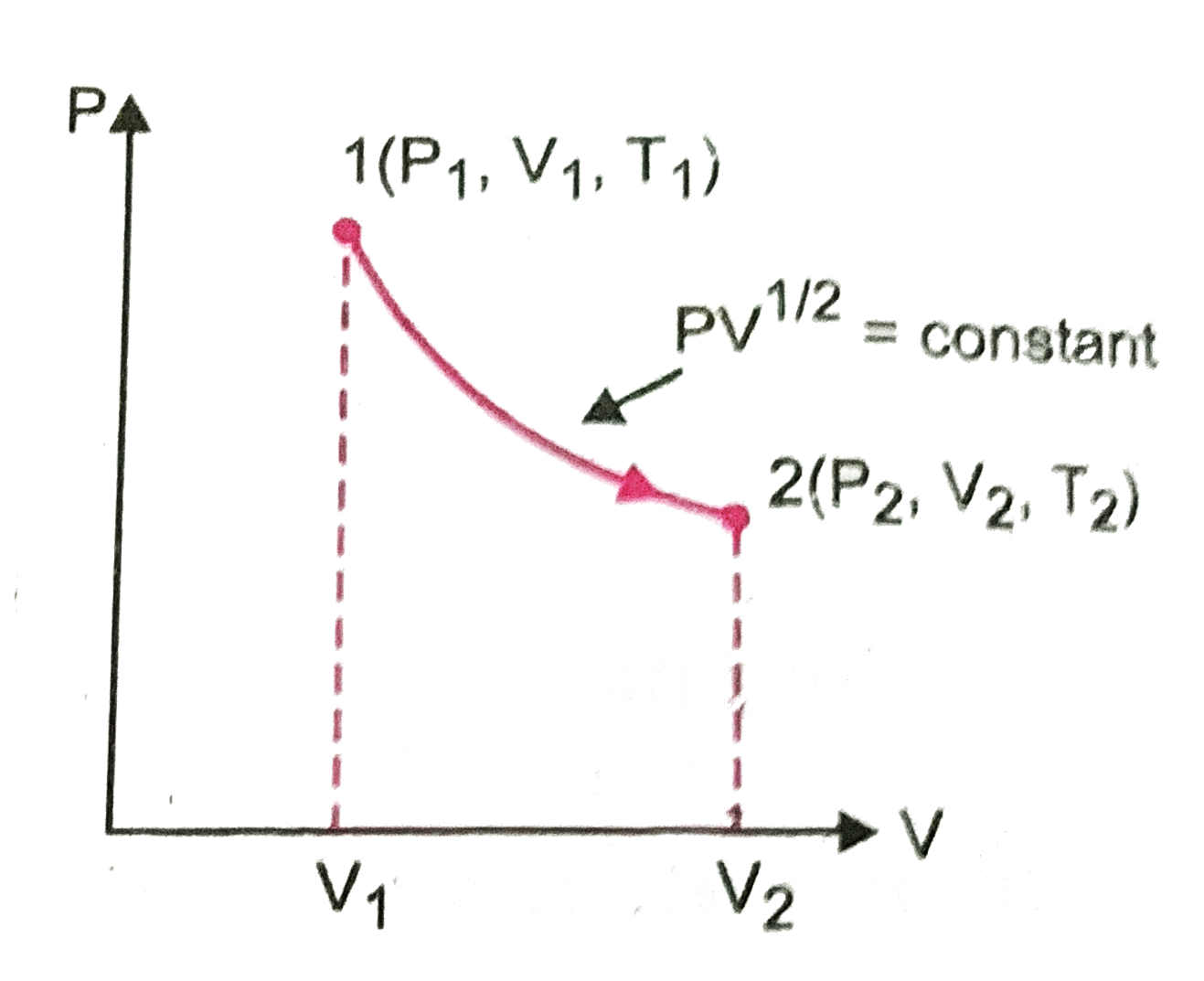
View Solutionn moles of an ideal gas is taken through a four step cyclic process as shown in the diagram. Calculate work done by the gas in a cycle in terms of temperatures T_(1), T_(2), T_(3) and T_(4)
View SolutionOne mole of an ideal gas at a temperature T1 expands according to the law pV= a constant.Findthe work donw when the final temperature is T2
View SolutionThe V - i graph for a conductor at temperature T_(1) and T_(2) are as shown in the figure. ( T_(2) - T_(1)) is proportional to
View SolutionTwo different adiabatic parts for the same gas intersect two isothermals at T1andT2 shown in P-V diagram . Then the ratio of VaVb will be
View Solution1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V2=4V1 then the ratio of temperatures T1T2 will be
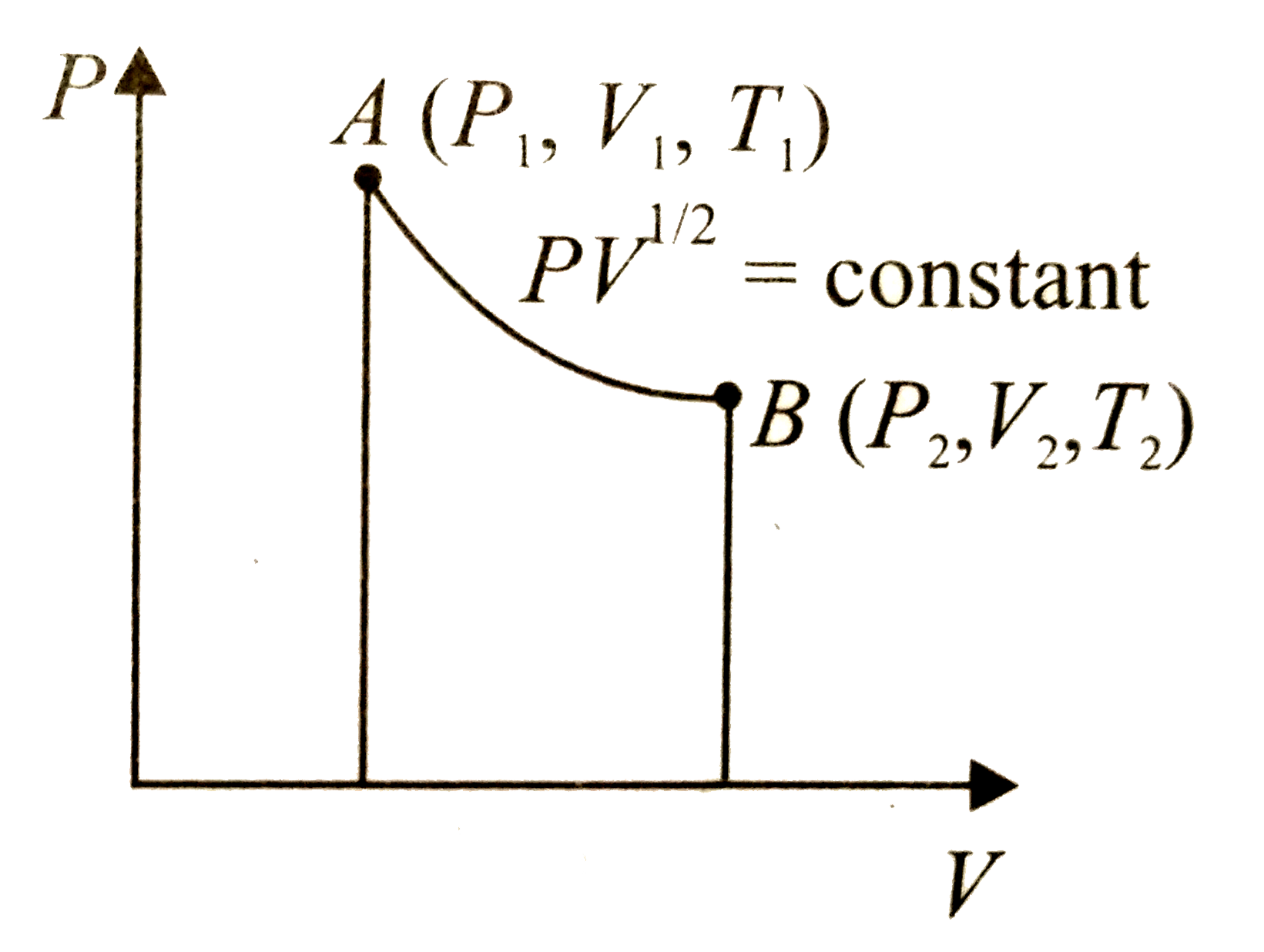
View SolutionThe velocity of sound waves in an ideal gas at temperatures T_(1) K and T_(2) are respectively v_(1) and v_(2) . The rms velocity of gas molecules at these two temperatures are w _(1) and e_(2) , respectively then
View SolutionConsider a P−V diagram in which the path followed by one mole of perfect gas in a cyclinderical container is shown in (figure)
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperatures T1/T2,ifV2=2V1?
(c ) Given the internal energy for one mole of gas at temperature Tis(3/2)RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1.
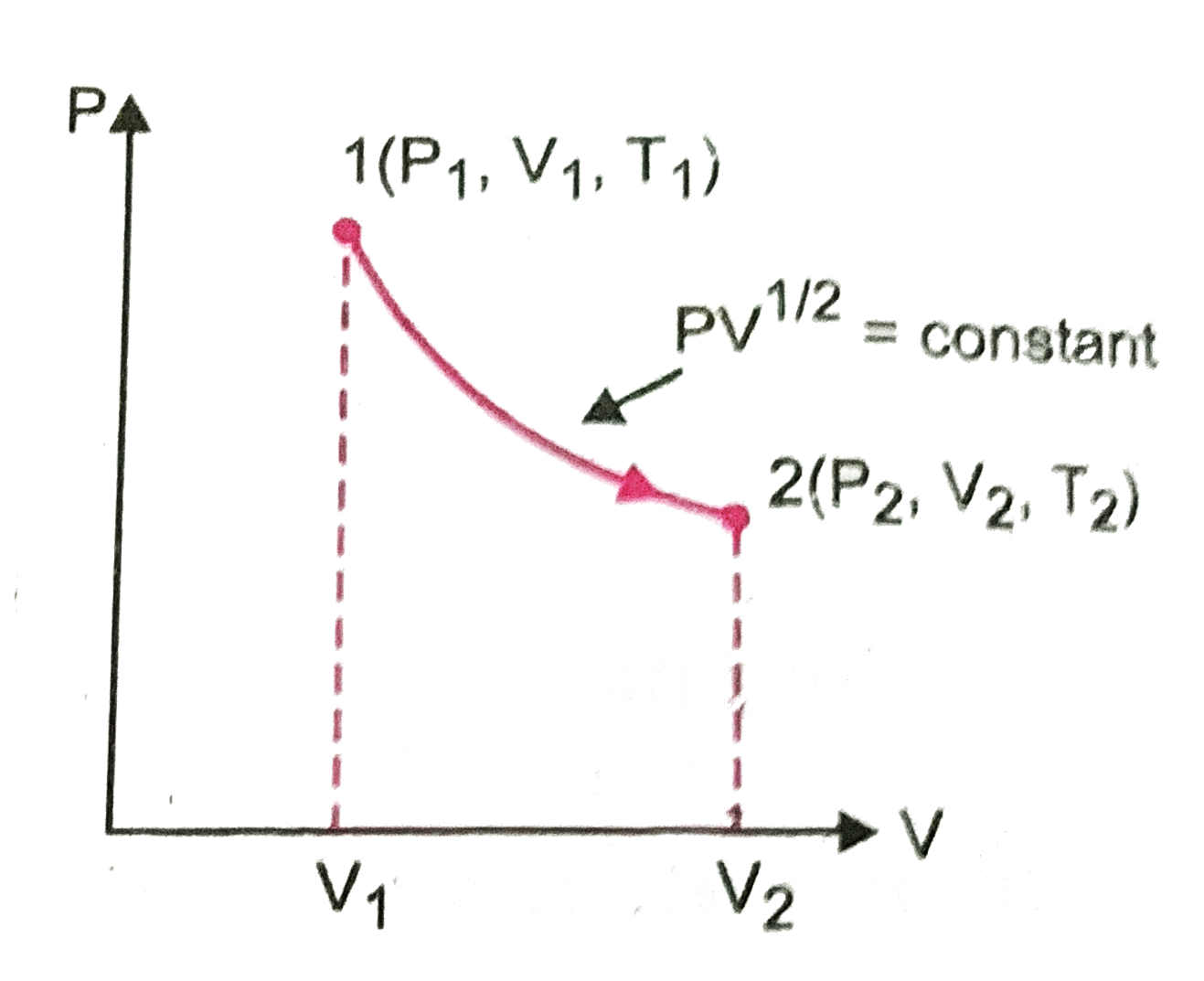
View SolutionThe velocities of sound in an ideal gas at temperature T1andT2 K are found to be V1andV2 respectively. If ther.m.s velocities of the molecules of the same gas at the same temperatures T1andT2 are v1andv2 respectively then
View SolutionThe V - i graph for a conductor at temperature T_(1) and T_(2) are as shown in the figure. ( T_(2) - T_(1)) is proportional to
View SolutionTwo different adiabatic parts for the same gas intersect two isothermals at T1andT_2 as shown in P-V diagram . Then the ratio of VaVb will be

View Solution1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure.If V_(2)=4V_(1) then the ratio of temperatures (T_(1))/(T_(2)) will be
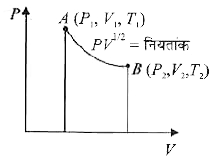
View Solutionबेलनाकार पात्र में किसी आदर्श गैस के 1 मोल के P-V आरेख को चित्रानुसार दर्शाया गया है। यदि V2=4V1, तो ताप का T1T2 अनुपात होगा-
View SolutionConsider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container as shown in Fig
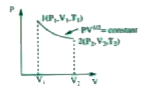
a) Find the work done when the gas is taken from state 1 to state 2.
b) What is the ratio of temperature T1/T2, if V2=2V1 ?
c) Given the internal energy for one mole of gas at temperature T is (3/2) RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1 .
View Solution

