Draw the geometrical isomers of above compound.
Topper's Solved these Questions
- HYDROCARBONSBook:KUMAR PRAKASHANChapter:HYDROCARBONSExercise:Section - B (Fill in the blanks)
Explore 12 Videos
- HYDROCARBONSBook:KUMAR PRAKASHANChapter:HYDROCARBONSExercise:Section - B (State True Or False For The Following Statements)
Explore 11 Videos
- HYDROCARBONSBook:KUMAR PRAKASHANChapter:HYDROCARBONSExercise:SECTION - A
Explore 55 Videos
- EQUILIBRIUMBook:KUMAR PRAKASHANChapter:EQUILIBRIUMExercise:Question Paper from Module
Explore 7 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
Similar Questions
Stronger deactivator group is ……… .
View SolutionBenzene is aromatic because ………
(i) it has six carbon.
(ii) all carbon has sp2 hybridization.
(iii) it has three C=C.
(iv) it has more proportion of carbon and less proportion of hydrogen.
(v) it is planner in nature.
View SolutionWrite structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also write their IUPAC names.
View SolutionAt 60∘H2O(l)−−→←−−H2O(g) in this equilibrium the value of Kp ? At 60∘C upper pressure of water is 0.185 bar.
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View Solution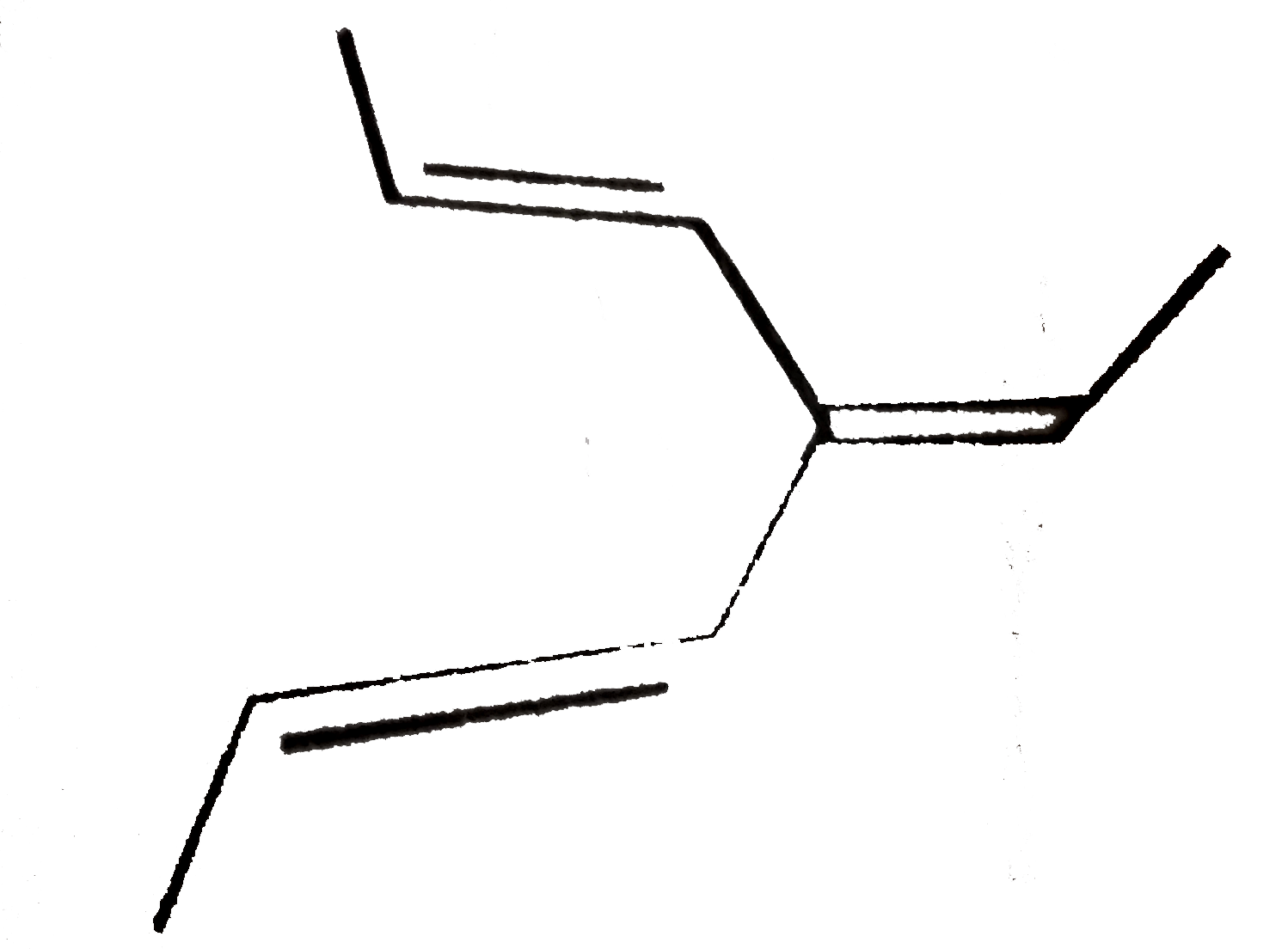
How many geometrical isomers are possible for the above compound ?
View Solution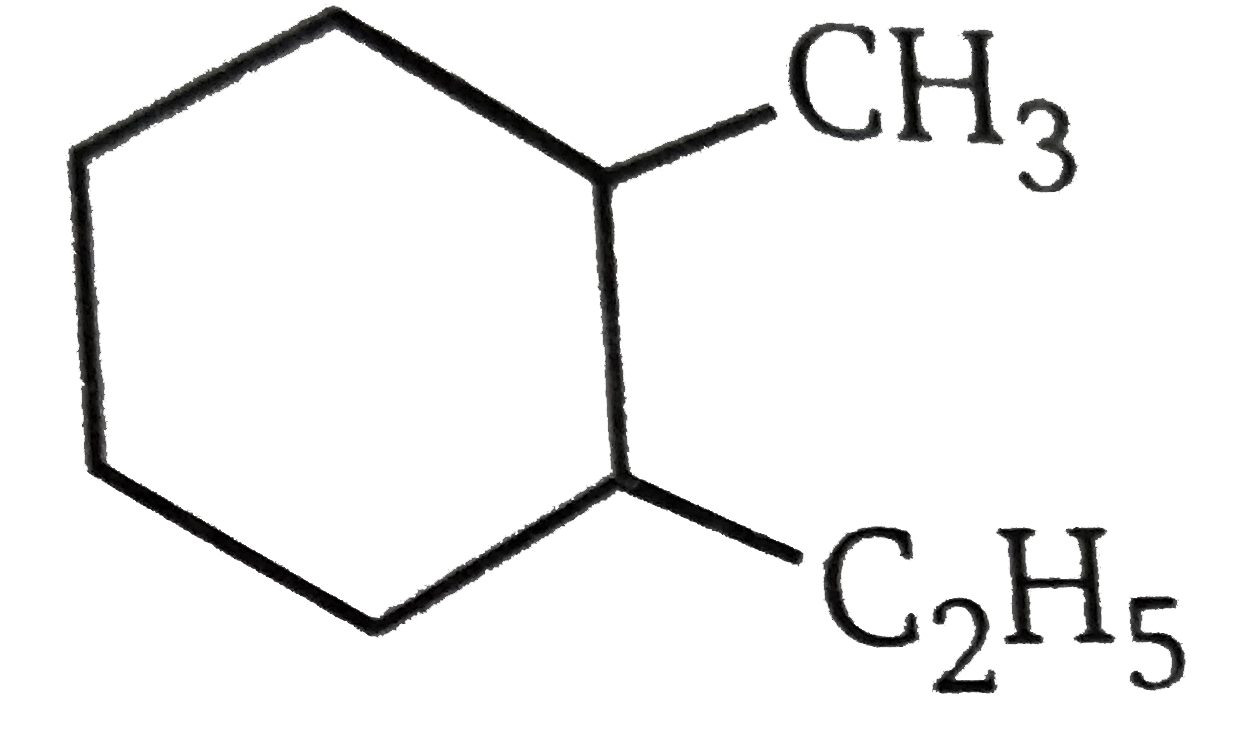
How many geometrical isomers are possible for the above compound ?
View Solution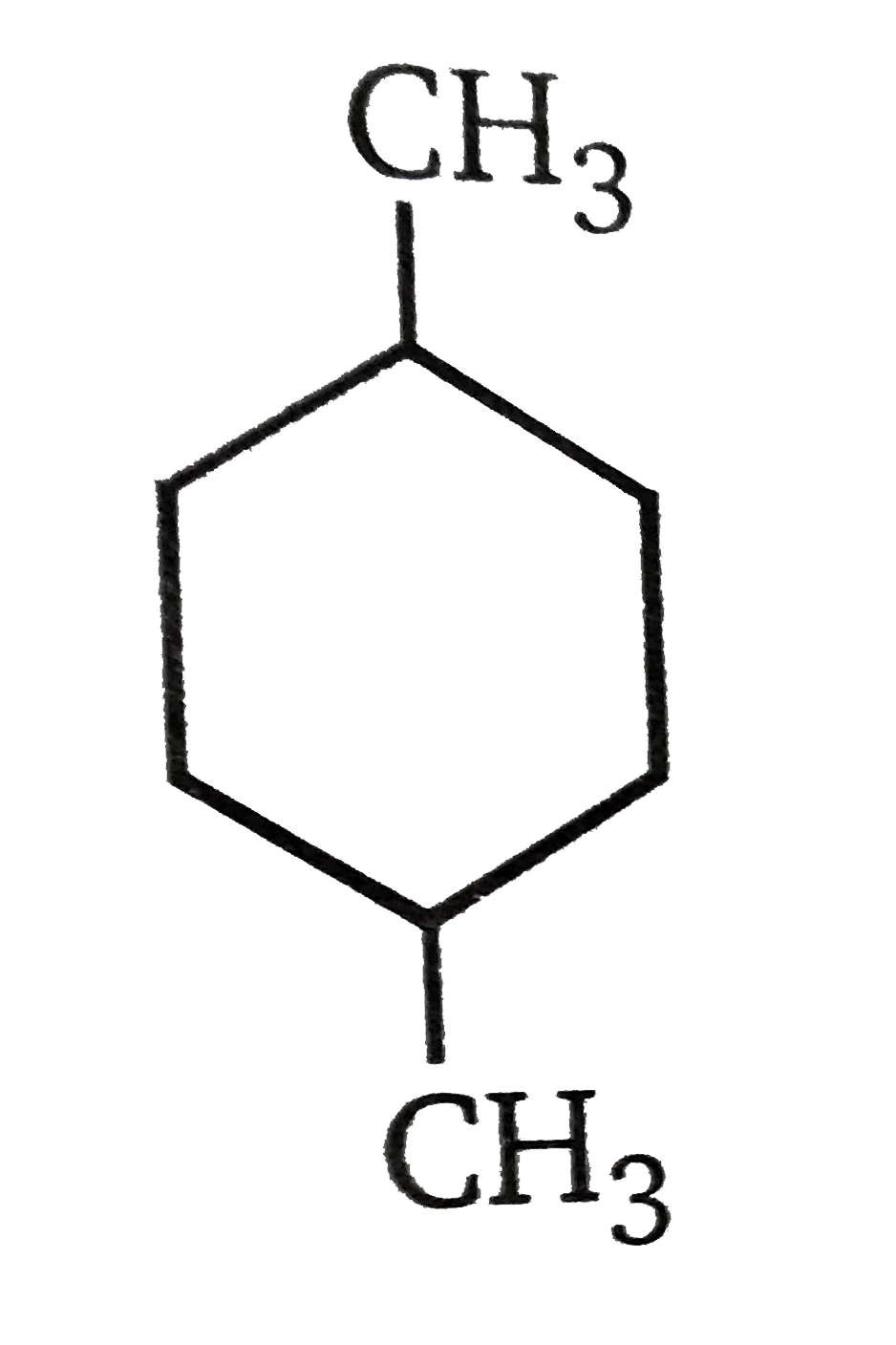
How many geometrical isomers are possible for the above compound ?
View Solution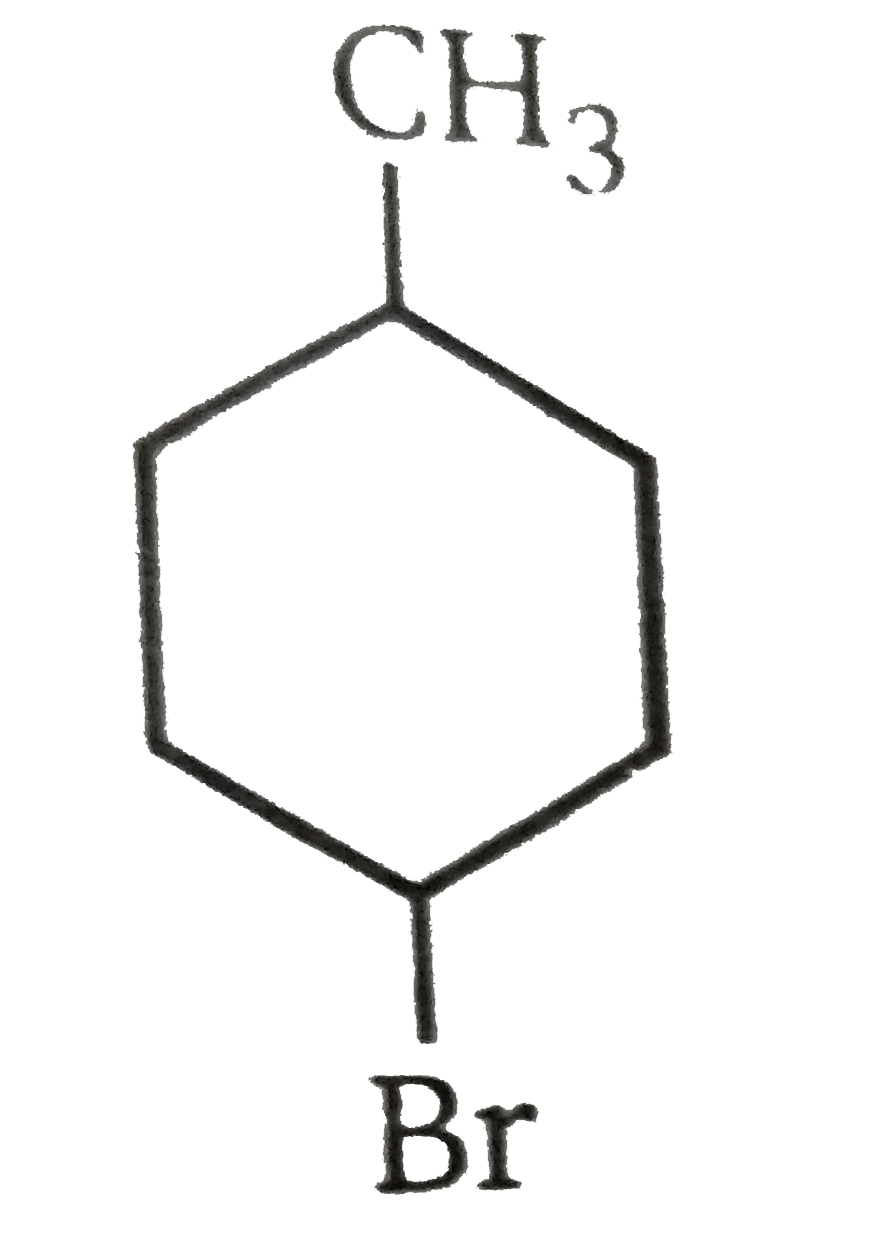
How many geometrical isomers are possible for the above compound ?
View Solution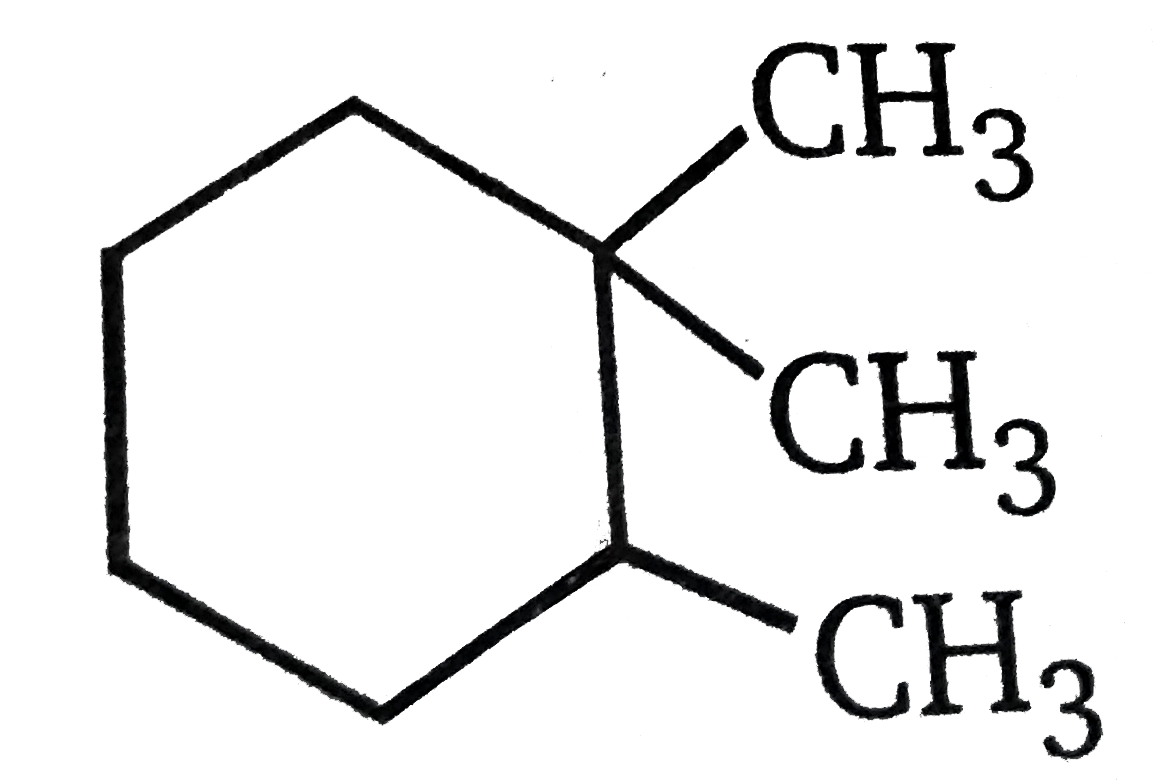
How many geometrical isomers are possible for the above compound ?
View Solution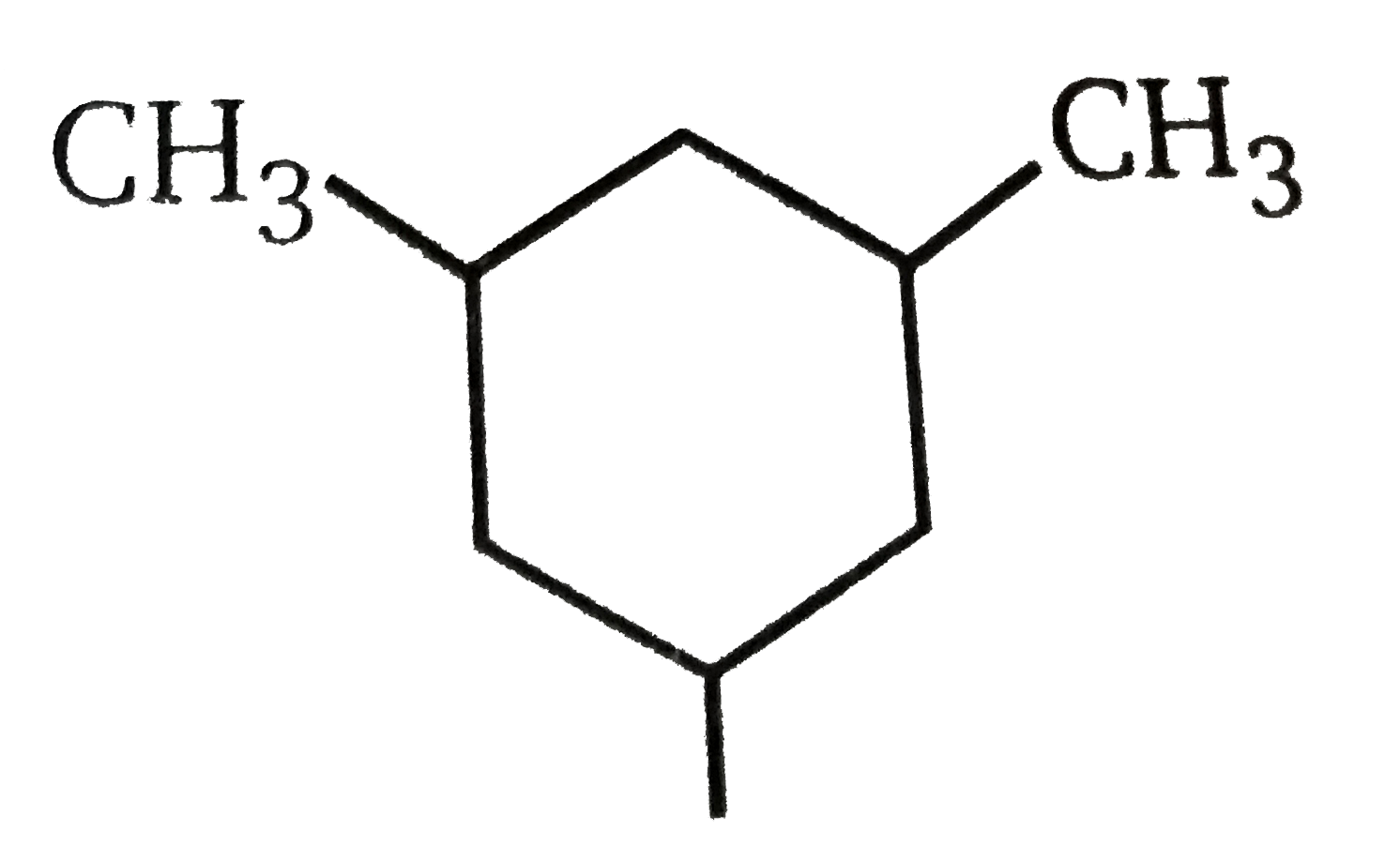
How many geometrical isomers are possible for the above compound ?
View SolutionNo. of Geometrical isomers for following compound is :
View Solution" Number of geometrical isomers for the below compound "
View SolutionDraw the structures of these geometrical isomers.
View SolutionDraw the two geometrical isomers of the complex compound [Pt(NH3)2Cl2]
View Solution


