The decreasing order of boiling points is
A
n-pentane > iso-pentane > neo-pentane
B
iso-pentane > n-pentane > neo-pentane
C
neo-pentane > iso-pentane > n-pentane
D
n-pentane > neo-pentane > iso-pentane
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
The decreasing order of boiling points of the following hydrides is
ASbH3>AsH3>PH3>NH3BNH3>SbH3>AsH3>PH3CSbH3>NH3>AsH3>PH3DPH3>AsH3>SbH3>NH3The decreasing order of boiling points of the following hydrides is
ASbH3>AsH3>PH3>NH3BNH3>SbH3>AsH3>PH3CSbH3>NH3>AsH3>PH3DPH3>AsH3>SbH3>NH3The decreasing order of boiling points of alkyl halides is
ARF>RCl>RBr>RIBRBr>RCl>RI>GFCRI>RBr>RCl>RFDRI>RF>RCl>RBr
The decreasing order of boiling points of the following is :
(I) RCOCl
(II) (RCO)2O
(III) RCONH2
(IV) RCOOH.
View SolutionThe decreasing order of boiling points of ethyldimethylamine, n-butylamine and diethylamine is n-Butylamine,Diethylamine,Ethyldimethylamine. This trend of boiling point can be explained as
View SolutionThe decreasing order of boiling points of the following compounds is:
(I)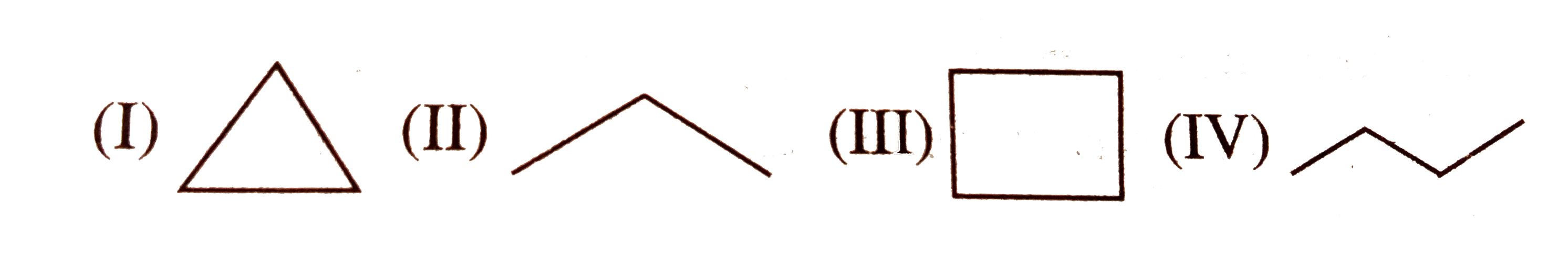
View SolutionThe decreasing order of boiling points of the following alcohols is
View SolutionThe decreasing order of boiling points of the following alcohols is
View Solution

