With a neat labelled diagram,describe the extraction of aluminium by Hall - Haroult process.Solution in Kannada
Labelled diagram
Explanation
2Al2O3+3C→4Al+3CO2
OR
Cathod : Al3++3e−→Al
Anode : C+O2+→CO+2e−0
OR
C+2O2−→CO2+4e−
Detailed Answer :
Extraction of aluminium from bauxte : .Al. is extracted from Bauxite (Al2O3.2H2O).
(i) Concentration of bauxite is done by leaching.
(ii) Electrolytic Reduuction (Hall -Heroult Process ) : Purified bauxite bauxite ore is mixed with cryolite (Na3AlF6)orCaF2 Whcih lowers its melting point and increases electrolysis is using graphite rods as anode and carbon lining as cathode.
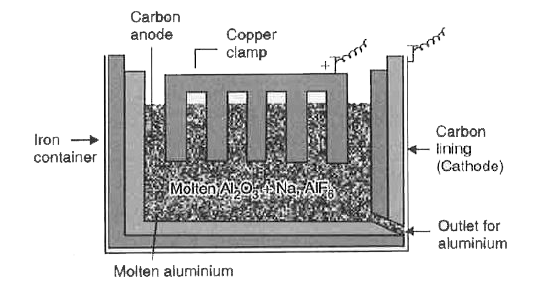
The graphite anode is useful for reduction of metal oxide to metal
2Al_2O_3 + 3C to 4Al +3CO_2<br>Al_2O_3 overset("electrolysis")to 2Al^(3+) + 3CO^(2-)<br>Atcathode:Al^(3+) ("melt") +3e^(-) to Al(l)<br>Atanode:O^(2-) - 2e^(-) to O<br>O +O to O_2<br>C +O_2 to CO_2<br>Graϕterods≥tburntformingCOandCO_2 the aluminium thus obtaned is refined electrolytically using impure Al anode, pure Al as cathode and molten cryolite as electrolyte.
Topper's Solved these Questions
- SOLVED PAPER II PUC JULY - 2016Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC JULY - 2016Exercise:PART -D
Explore 13 Videos
- SOLVED PAPER II PUC JULY - 2016Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC JULY - 2016Exercise:PART -E
Explore 19 Videos
- SOLVED PAPER II PUC JULY - 2016Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC JULY - 2016Exercise:PART -B
Explore 10 Videos
- SOLVED PAPER II PUC APRIL-2016 Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC APRIL-2016 Exercise:PART-D
Explore 27 Videos
- SOLVED PAPER II PUC MARCH-2016 Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC MARCH-2016 Exercise:PART-D
Explore 24 Videos
Similar Questions
Calculate packing efficiency in BCC lattice.
View SolutionExplain SN2 mechanism taking an example of chloromethane.
View SolutionWhat type of stoichiometric defect is shown by AgCl ?
View Solutiona. Calculate the packing efficiency in a Body Centered Cubic (BCC) lattice.
b. Silver forms a ccp lattice. The edge length of its unit cell is 408.6 pm. Calculate the density of silver. (NA=6.022×1023, Atomic mass of Ag =108gmol−1)
View Solution200cm3 of an aqueous solution of a protein contains 1.26 g of protein. The osmotic pressure of such a solution at 300 K is found to be 2.57×10−3 bar. Calculate the molar mass of the protein.
(R = 0.083 L bar mol−1K−1)
View SolutionDraw a neat, labelled diagram of electrolytic cell for the extraction of aluminium.
View SolutionDraw a neat, labelled diagram of electrolytic cell for the extraction of aluminium.
View SolutionExplain the method used for the extraction of aluminium from its ore. Illustrate your answer with the help of a neat, labelled diagram.
View SolutionDescribe the electrolytic reduction of alumina with neat labelled diagram
View SolutionDescribe the process of Land filling site with a neat labelled diagram.
View SolutionDescribe the internal stsructure of heart with a neat labelled diagram.
View SolutionWith a neat labelled diagram,describe the extraction of aluminium by Hall - Haroult process.
View SolutionDraw labelled diagram of Hall-Heroult electrolytic cell for the extraction of aluminium write anode and cathode reactions.
View SolutionIn the extraction of Aluminium by Hall- Herault process:
Give the equation of overall cell reaction.
View SolutionWith the help of neat labelled diagram describe human sperm.
View Solution
