The compressibility factor ( z) for a real gas at its Boyle temperature is _____________.Solution in Marathi
1
0
>1
<1
The correct Answer is:A
Step by step video & image solution for The compressibility factor ( z) for a real gas at its Boyle temperature is "_____________". by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
The compressibility factor ( z) for a real gas at its Boyle temperature is _____________.
View SolutionAt Boyle temperature , what is the value of compressibility factor of a real gas ?
View SolutionCompressibility factor, Z of a gas is given as Z= PV"/"nRT For real gas, what is the value of Z above Boyle's temperature?
View SolutionCompressibility factor (Z) is plotted against pressure at different temperature for same gas
View SolutionAt the temperature of the Boyle, the compressive coefficient Z for the real gas is-
View SolutionBoyle's temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between compressibility factor Z and pressure P.
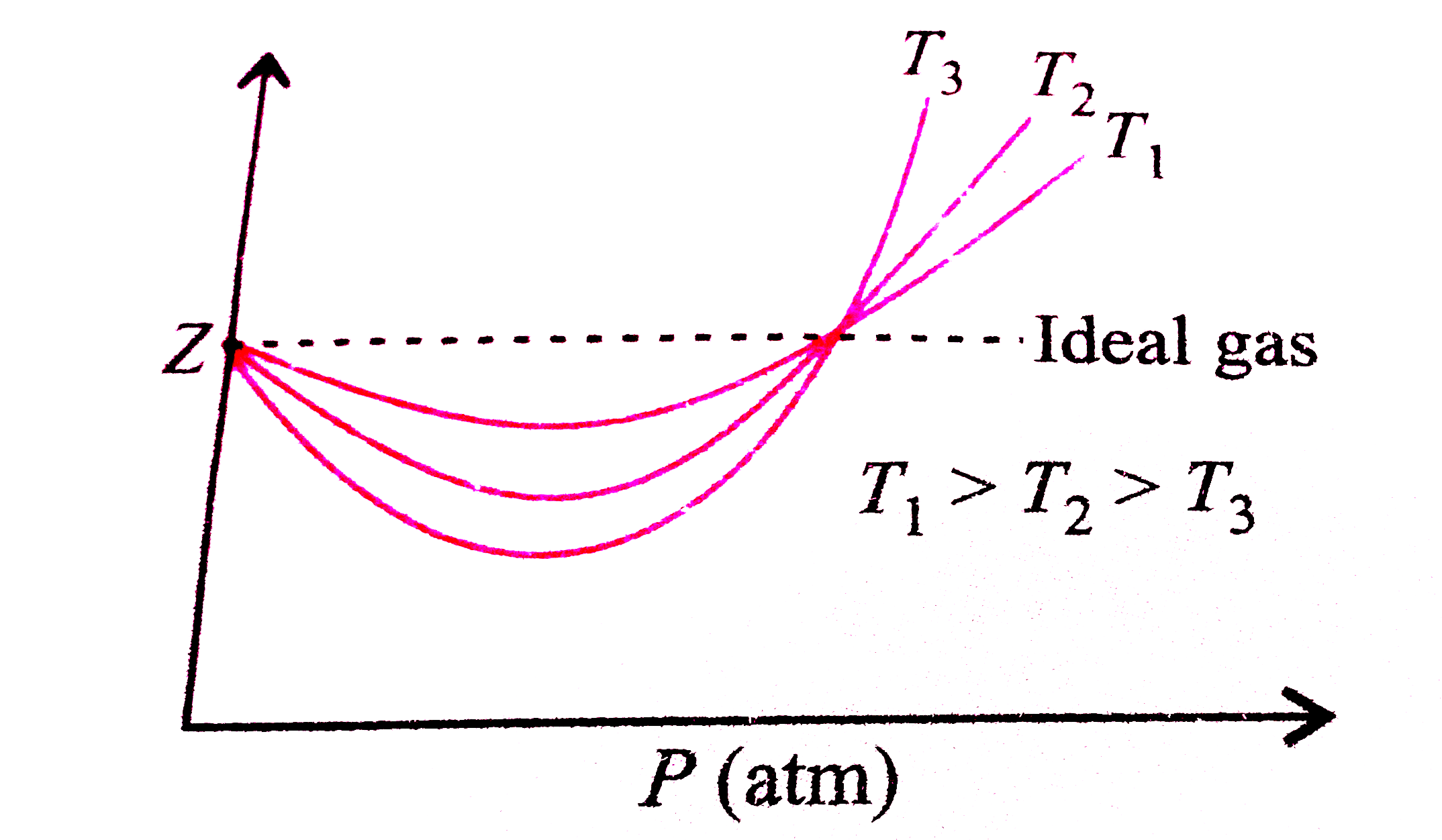
What is the deviation of real gas from ideal behaviour in terms of compressibility factor , Z ?
View SolutionAt Boyle's temperature , compressibility factor Z for a real gas is
View Solution

