Which statement is not true for astatine:Solution in Odia
It is less electronegative than iodine
It exhibits only-1 oxidation state
Intermolecular forces between the astatine molecules will be larger than between the iodine molecules.
It is composed of diatomic molecules.
The correct Answer is:B
Step by step video, text & image solution for Which statement is not true for astatine: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Write the IUPAC name of following compound.
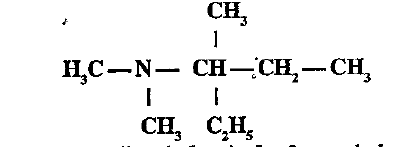
View SolutionWrite two uses of Bakelite ?
View SolutionAstatine is the element below iodine in the group VIIA of the periodic table. Which of the following statements is not true for astatine?
View SolutionAstatine is a radioactive halogen. It is a solid at room temperature because
View SolutionAstatine is the element below iodine in the group 17 of the periodic table. Which of the following statement is not true for Astatine?
View SolutionAstatine is a
View SolutionIf p, q are true and r is false statement then which of the following is true statement?
View SolutionWhich of the following statements are true statements :
View SolutionThe aotmic number of astatine which belongs to six period is
View SolutionAstatine is the element below iodine in the group 17 of the periodic table. Which of the following statements (s) is ( are ) true for astatine ?
View SolutionWhich of the halogens (excluding astatine) has highest electronegativity ?
View SolutionWhich of the halogens (excluding astatine) is strongest oxidant ?
View SolutionWhich of the halogens (excluding astatine)has lowest ionisation energy ?
View SolutionAstatine is the element below iodine in the group VIIA of the periodic table. Which of the following statements is not true for astatine?
View Solutionनिम्न कथनों में से सत्य कथन है
View SolutionThe symbol for the element Astatine is
View Solution

