Fluorine is a very strong oxidising agent:Solution in Odia
It has several isotopes
It is very small and has seven electrons in its valency shell
It is the first member of the halogen series
Its valency is one.
The correct Answer is:B
Step by step video, text & image solution for Fluorine is a very strong oxidising agent: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Write the IUPAC name of following compound.
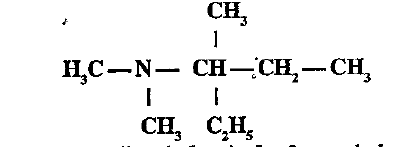
View SolutionWrite two uses of Bakelite ?
View SolutionThe strong oxidising agent has :
View SolutionExplain on the basis of standard reduction potentials, fluorine is a strong oxidising agent while potassium is a strong reducing agent.
View SolutionFluorine is a powerful oxidising agent. Why?
View SolutionGlucose is oxidised by strong oxidising agent gives
View SolutionStrong oxidising agent oxidised propanone to give
View Solutionफ्लुओरिन , क्लोरीन की तुलना में प्रबल ऑक्सीकारक क्यों है ?
View Solutionप्रबल ऑक्सीकारक है
View SolutionThe SRP of a strong oxidising agent is
View SolutionWhich is the strongest oxidising agent between chlorine and fluorine?
View SolutionAcidified KMnO_(4) is a very strong oxidising agent. Prove it.
View SolutionAssertion (A) : F2 is a strong oxidising agent.
Reason (R ) : Electron gain enthalpy of fluorine is less negative.
View SolutionWhich of the following factors is responsible for the property of fluorine as strong oxidising agent ?
View Solution(A) : Fluorine is the best oxidising agent
(R ) SRP is highest for Fluorine
View SolutionFluorine is the best oxidising agent because it has
View Solution

