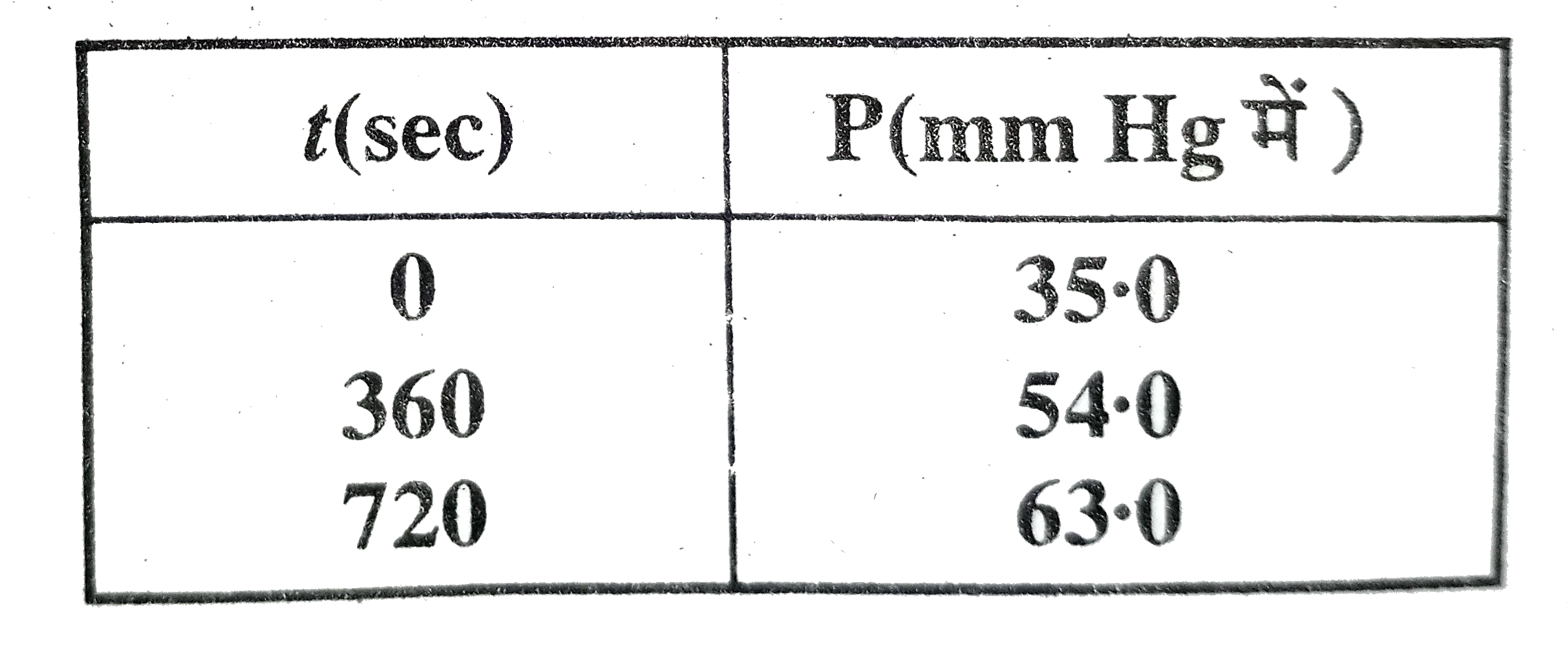
For the decomposition of azoisopropane to hexane and nitrogen at 543K, the following data are obtained:
SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate constant.
(CH3)2CHN=NCH(CH3)2(g)→N2(g)+C6H14(g)Initial pressure P000Pressure after time t P0−ppp
Total Pressure after time t,
Pt=(P0−p)+p+p=P0+porp=Pt−P0
a∝P0and(a−x)∝P0−P
Substituting the value of p, we get
a−x∝P0−(Pt−P0),i.e.,(a−x)∝2P0−Pt
As decomposition of azoisopropane is a first order reaction
k=2.303tlogaa−x=2.303tlogP02P0−Pt(usingP02P0−Pt in place of aa−x)
At t = 360 seconds, k=2.303360slog35.02×35.0−54.0=2.303360slog3516
=2.303360s(0.3400)=2.175×10−3s−1
At t = 720 seconds, k=2.303720slog35.02×35.0−63.0=2.303720slog5
=2.303720s(0.6990)=2.235×10−3s−1
∴ Average value of k=2.175+2.2352×10−3s−1=2.20×10−3s−1.
Topper's Solved these Questions
- CHEMICAL KINETICS BOOK - U-LIKE SERIESCHAPTER - CHEMICAL KINETICS EXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS
15 Videos
- CHEMICAL KINETICS BOOK - U-LIKE SERIESCHAPTER - CHEMICAL KINETICS EXERCISE - MULTIPLE CHOICE QUESTIONS
19 Videos
- CHEMICAL KINETICS BOOK - U-LIKE SERIESCHAPTER - CHEMICAL KINETICS EXERCISE - SELF ASSESSMENT TEST (SECTION D)
1 Video
- CBSE EXAMINATION PAPER 2020 (CODE No.56/4/1) BOOK - U-LIKE SERIESCHAPTER - CBSE EXAMINATION PAPER 2020 (CODE No.56/4/1) EXERCISE - SECTION -D
3 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - U-LIKE SERIESCHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - LONG ANSWER QUESTIONS - I
21 Videos
Similar Questions
Read the given passage and answer the questions:
Zero order reactions are relatively uncommon but they occur under special conditions. Some enzyme catalysed reactions and reactions which occurs on metal surfaces are a few examples of zero order reactions. The decompositions which occurs on metal surfaces are a few examples of zero order reactions. The decomposition of gaseous ammonia on a hot platinum surface is a zero order reaction at high pressure.
2NH3(g)1130K−−−−−→Pt catalystN2(g)+3H2(g)
Rate=k[NH3]0=k
In this reaction, Pt acts as a catalyst. At high pressure, the metal surface gets saturated with gas molecules. So a further change in reaction conditions is unable to alter the amount of ammonia on the surface of the catalyst making the rate of the reaction independent of its concentration.
Q. What are zero order reactions?
View SolutionUnits of rate constant for a second order reaction is
View Solution(a) Explain the following terms:
(i) Rate of a reaction. (ii) Activation energy of a reaction.
(b)The decomposition of phosphine, PH3 proceeds according to the following equation:
Rate =k[PH3]
The half-life of PH3 is 37.9 s at 120∘C.
(i) How much time is required for 3/4th of PH3 to decompose?
(ii) What fraction of the original sample of PH3 remains behind after 1 minute?
View Solution(a) The electrical resistance of a column of 0.05MKOH solution of length 50 cm and area of cross-section 0.625cm2 is 5×103 ohm. Calculate its resistivity, conductivity and molar conductivity.
(b) Predict the products of electrolysis of an aqueous solution of CuCI2 with platinum electrodes.
[Given : EoCu2+/Cu=+0.34V,Eo(12CI2/CI−)=+1.36V,EoH+/H2(g),Pt=0.00V,E(12O2/H2O)=+1.23V]
OR
(a) Calculate e.m.f. of the following cell :
Zn(s)/Zn2+(0.1M)∣∣(0.01M)Ag+/Ag(s)
[Given : EoZn2+/Zn=−0.76V,EoAg+/Ag=+0.80V,log10=1]
(b) X and Y are two electrolytes. On dilution molar conductivity of 'X' increases 2.5 times while that Y increases 25 times. Which of the two is a weak electrolyte and why ?
View Solution(a) Why is bithional added to soap ?
(b) What is tincture of iodine ? Write its one use.
(c) Among the following, which one acts as a food preservative ?
Aspartame, Aspirin, Sodium Benzoate, Paracetamol
View SolutionThe following data were obtained during the first order thermal decomposition of SO_(2)Cl_(2) at a constant volume SO_(2)Cl_(2)(g)to SO_(2)(g)+Cl_(2)(g) Calculate the rate of the reaction when total pressure is 0.65 atm
View SolutionFor the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data is obtained. Calculate the rate constant.
View SolutionThe following data were obtained during first order thermal decomposition of SO2(Cl2) at a constant volume.
SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate of the reaction when the total pressure is 0.65 atm.
View SolutionThe following data were obtained during the first order decomposition of SO_(2)Cl_(2) at a constant volume: SO_(2)Cl_(2)(g) to SO_(2)(g) + Cl_(2)(g) Calculate the rate constant.
View SolutionThe following data were obtained during the first order thermal decomposition of SO_(2) Cl_(2) at a constant volume : SO_(2)Cl_(2) (g) to SO_(2)(g)+Cl_(2)(g) {:("Experiment Times"//s^(-1) " Total pressure/atm"),("1 0 0.4" ),("2 100 0.7"):} Calculate the rate constant. Given : log 4 = 0.6021, log 2 = 0.3010)
View Solution543K ताप पर एजोआइसोप्रोपेन के हेक्सेन तथा नाइट्रोजन में विघटन के निम्न आँकड़े प्राप्त हुए। वेग स्थिरांक की गणना कीजिए-
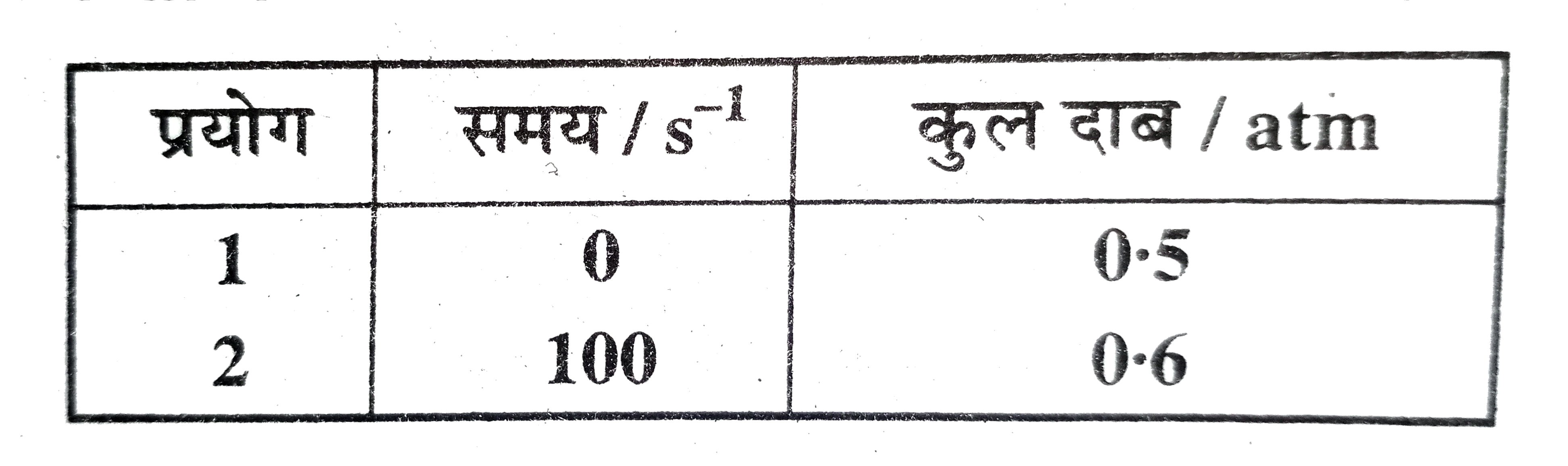
View Solutionस्थिर आयतन पर, SO2Cl2 के प्रथम कोटि के ताप अपघटन पर निम्न आँकड़े प्राप्त हुए-
SO2Cl2(g)→SO2(g)+Cl2(g)
अभिक्रिया वेग की गणना कीजिए जब कुल दाब 0.65 atm हो-
View SolutionFollowing data were obtained during first order thermal decomposition of - SO_(2)Cl_(2)(g)rarrSO_(2)(g)+Cl_(2)(g) {:("Experiment","time"(s^(-1)),"Total pressure"("atm")),(1,0,0.4),(2,100,0.7):} Calculate the rate constant . (Given : log4 = 0.6021, log2 = 0.3010)
View SolutionFor the first order decomposition of SO_(2)Cl_(2)(g), SO_(2)Cl_(2)(g) rarr SO_(2)(g) + Cl_(2)(g) a graph of log (a_(0) – x) vs t is shown in figure. What is the rate constant (sec^(–1)) ?
View SolutionThe following data were obtained during the first order thermal decomposition of SO_(2)Cl_(2) at a constant volume. SO_(2)Cl_(2(g))rarr SO_(2(g))+Cl_(2(g)) What is the rate of reaction when total pressure of 0.65 atm?
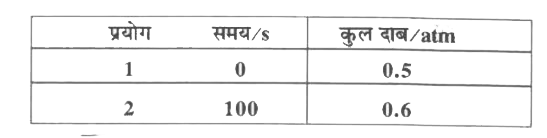
View Solutionस्थिर आयतन पर SO2Cl2 के प्रथम कोटि के ताप अपघटन पर निम्न आँकड़े प्राप्त हुए-
SO2Cl2(g)→SO2(g)+Cl2(g)
अभिक्रिया वेग की गणना कीजिए जब कुल दाब 0.65 हो।
View Solution

