Explain close packing in two dimensions.
The close packing in two dimensions can be done in two ways by stacking the rows of closed packed spheres : (i) Square Close Packing (ii) Hexagonal Close Packing (HCP)
(i) Square Close Packing : In square close packing, the spheres of second row are placed exactly above the spheres of first row such that spheres of these two rows are aligned horizontally as well as vertically.
If the first row of spheres is called "A" type row, the second row being exactly the same as the first one is also of ..A.. type and hence by placing more rows, AAAA..... type of arrangement is obtained.
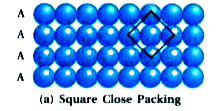
In this arrangement, each sphere is in contact with four of its neighbours and hence the coordination number of a sphere is four.
If the centres of the four immediate neighbours are joined a square is formed and hence it is named Square Closed Packing.
(ii) Hexagonal Closed Packing (HCP) :

In hexagonal close packing, the spheres of second row are fit in the depressions of the spheres of first row.
If the spheres of first row is called ..A.. type, the one in second row may be called ..B.. type.
The spheres of third row are placed in depressions of the spheres of second row such that spheres of first row and third row are in same alignment horizontally and vertically.
As the third row is exactly same as that of first row it is called ..A.. type. Hence, we get ABABAB .... type of arrangement.
In this arrangement each sphere is in contact with six of its neighbour and thus in two dimensional the co-ordination number is six. The centres of these six spheres are at the corners of regular hexagon hence this packing is called two dimensional hexagonal close packing.
In this arrangement, there is less free space and so this packing is more efficient than square close packing.
The empty spaces called voids are surrounded by three spheres in this arrangement. The centres of these three touching spheres form a triangle and hence it is called Triangular Voids. In one row, the apex of the triangles are pointed upwards and in the next layer dowards. Hence, we get two types of triangular voids.
Topper's Solved these Questions
- THE SOLID STATEBook:KUMAR PRAKASHANChapter:THE SOLID STATEExercise:SECTION - A (EXAMPLES)
Explore 5 Videos
- THE SOLID STATEBook:KUMAR PRAKASHANChapter:THE SOLID STATEExercise:SECTION - B (INTEXT QUESTIONS AND ANSWERS)
Explore 23 Videos
- THE SOLID STATEBook:KUMAR PRAKASHANChapter:THE SOLID STATEExercise:SECTION - E (MULTIPLE CHOICE QUESTIONS)(MCQs ASKED IN BOARD EXAMS)
Explore 35 Videos
- THE P-BLOCK ELEMENTS Book:KUMAR PRAKASHANChapter:THE P-BLOCK ELEMENTS Exercise:SECTION-E (MULTIPLE CHOICE QUESTIONS (MCQS)) (MCQS ASKED IN BOARD EXAM)
Explore 77 Videos
Similar Questions
A compound is formed by two elements X and Y. Atoms of the element Y (as anions) make ccp and those of the element X (as cations) occupy all the octahedral voids. What is the formula of the compound ?
View SolutionWhy are solids rigid ?
View SolutionWhich of the following solids has highest conductivity of heat and electricity ?
View SolutionWhat is the product of reaction between excess xenon and fluorine at 673 K temperature ?
View SolutionWhat is the coordination number in a square close packed structures in two dimensions?
View SolutionWhat is the coordination number in a square close packed structures in two dimensions?
View SolutionIn two dimensions, which one represents the close-packed structure of spheres with highest packing efficiency, a square close-packing or a hexagonal close-packing? Explain in it terms of coordination number of sphere in each case.
View SolutionWhat is the coordination number in a square close-packed structure in two dimensions-
View SolutionWhat is the coordination number in a square close packed structures in two dimensions?
View SolutionWhat is the coordination number in a square close packed structures in two dimensions?
View Solutionexplain close packing in one dimension.
View SolutionExplain hexagonal close packing in three dimensions.
View SolutionWhich are the two types of close packing in two dimension. What are its differences?
View SolutionExplain close packing in one dimension.
View SolutionWhat is the coordination number in a square close packed structure in two dimensions ?
View Solution

