Draw the graph for [R] versus time (t) for a zero order reaction. Give the relationship between the rate constant and the slope of the curve. Solution in Kannada
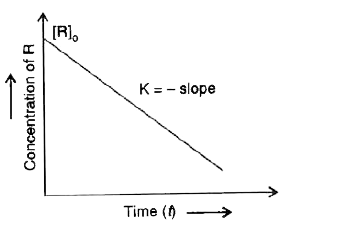
K=[R]0−[R]t
Topper's Solved these Questions
- SOLVED PAPER II PUC MARCH-2016 Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC MARCH-2016 Exercise:PART-C
Explore 11 Videos
- SOLVED PAPER II PUC JULY - 2016Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC JULY - 2016Exercise:PART -E
Explore 19 Videos
- SOLVED PAPER II PUC TOPPER'S ANSWER MARCH-2015Book:OSWAAL PUBLICATIONChapter:SOLVED PAPER II PUC TOPPER'S ANSWER MARCH-2015Exercise:PART -D
Explore 30 Videos
Similar Questions
For the manufacture of Ammonia by Haber's process, write the equation and optimum conditions for maximum yield of ammonia.
View SolutionExplain SN2 mechanism taking an example of chloromethane.
View SolutionCaleulate the packing efficiency in a unit cell of Cubic Close Packing(CCP) structure.
View SolutionThe relationship between rate constant and half life period of zero order reaction is give by
View Solutionप्रथम कोटि अभिक्रिया के लिए log10C तथा समय के मध्य आरेखित ग्राफ एक सीधी रेखा है। इस ग्राफ की प्रवणता तथा अभिक्रिया के वेग सिथरांक में क्या सम्बन्ध है?
View SolutionThe relationship between rate constant and half life period of zero order reaction is give by
View SolutionThe slope of a graph l n [At] versus t for a first order reaction is −2.5×10−3s−1 . The rate constant for the reaction will be
View SolutionA slope of a graph In [A] versus 't' for a first order is −5×10−4s−1. The rate constant for the reaction will be.
View Solutionकिसी प्रथम कोटि अभिक्रिया के लिए log[R] तथा समय (t) के मध्य ग्राफ का ढाल −2.2×10−5s−1 है तो इसके लिए वेग स्थिरांक क्या होगा?
View Solutionशून्य कोटि की अभिक्रिया के लिए समय तथा सान्द्रता के मध्य ग्राफ खींचिए।
View SolutionThe slope of the kinetic energy versus position vector curve gives the rate of change of :-
View SolutionFor a zero order reaction :
A→B
a graph of rate vs . Time has slope equal to :
View SolutionWhat is the value of slope of graph of concentration of R versus time for zero order reaction?
View SolutionDraw a plot of In[R] versus t for a first order reaction. What is slope of the line equal to?
View SolutionA plot of reactant concentration versus time for a reaction is a straight line with a negative slope giving the rate constant, and the intercept, giving the initial concentration of the reactant. The order of the reaction is
View Solution
