4 A neutral, resolvable organic compound A has molecular formula (C8H16O2). A on treatment with LiAlH4 gives isomeric B and C(C4H10O) of which only B is optically active. B on treatment with acidified dichromate solution gives D(C4H8O), which on refluxing with dilute NaOH followed by acidification of product gave E(C8H14O). E on heating with N2H4 in alkaline medium affords F(C8H16) which on treatment with B.H/H,O,/HO produced a resolvable G (C8H18O). G on treatment with acidified dichromate solution produced H(C8H16O), which on treatment with MCPBA produces A.
Compound (B) is

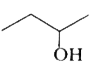
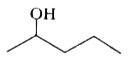
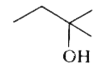
The correct Answer is:B
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:MS CHOUHANChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:ADDITIONAL OBJECTIVE QUESTIONS (Matrix Match Type)
Explore 1 Video
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:MS CHOUHANChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:ADDITIONAL OBJECTIVE QUESTIONS (SINGLE CORRECT CHOICE TYPE)
Explore 18 Videos
- CARBOXYLIC ACID AND THEIR DERIVATIVES Book:MS CHOUHANChapter:CARBOXYLIC ACID AND THEIR DERIVATIVES Exercise:Subjective Problems
Explore 1 Video
- CONJUGATED UNSATURATED SYSTEMSBook:MS CHOUHANChapter:CONJUGATED UNSATURATED SYSTEMSExercise:Additional Objective Questions (Multiple Correct Choice Type)
Explore 4 Videos
Similar Questions
View SolutionChoose the structure that is an intermediate in the base catalyzed hydrolysis of acetonitrile into acetamide.
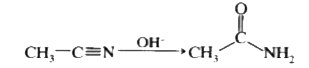
View Solution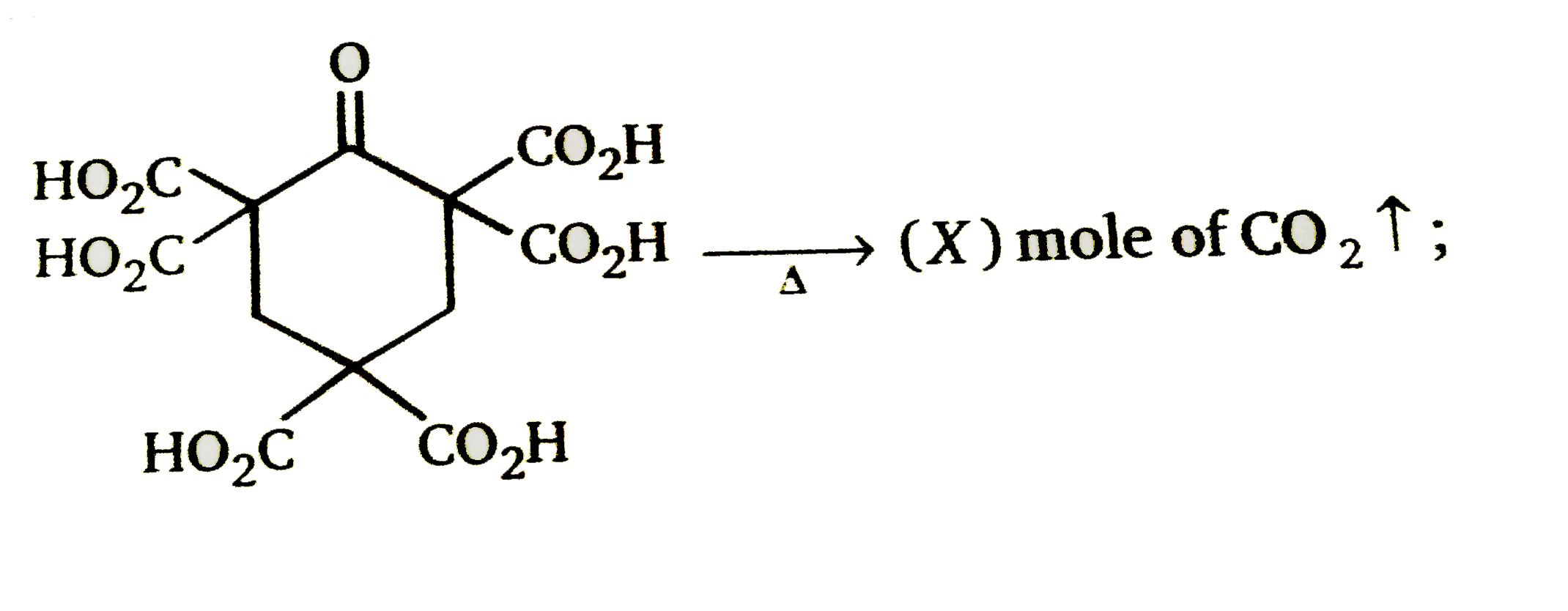
(Y) is including stereoisomers. Value of (X+Y) will be
View SolutionConsider the given reaction and identify the product P
CH3−CH=CH−CH2−OHHBr−−→SN1P
View SolutionGive the structures of (A),(B) and (C)(explanation is not required ).
i. (A)(C4H8) that adds on HBr in the presence and in the absence of peroxide to give the same product, C4H9Br.
ii. (B)(C4H8), which when treated with H2O/H2SO4 gives C4H10O that cannot be resolves into optical isomers.
iii. (C)(C6H12, an optically active hydrocarbon which on catalytic hydrogenation gives an optically inactive compound, C6H14)
View SolutionCompound (A) C8H6O2 on treatment with aq. NaOH followed by acidification gives (B) C8H8O3 which on oxidation gives benzoice acid only :
View SolutionAn organic compound A (C_(7)H_(6)Cl_(2)) on treatment with NaOH solution gives another compound B (C_(7)H_(6)O) . B on oxidation gives and acid C (C_(7)H_(6)O_(2)) which on treatment with a mixture of conc. HNO_(3)" and "H_(2)SO_(4) gives a compound D (C_(7)H_(5)NO_(4)) . B on treatment with conc. NaOH gives a compound E (C_(7)H_(8)O)" and "C_(6)H_(5)COONa . Deduce the structures of [A], [B], [C], [D] and [E].
View SolutionA substance C_(4)H_(10)O yields on oxidation a compound C_(4)H_(8)O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H_(2)SO_(4) gives C_(4)H_(8) , The structure of the compound is
View SolutionA substance C4H10O yields on oxidation a compound C4H8O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H2SO4 gives C4H8, The structure of the compound is
View SolutionAn organic compound [A] C6H10, on reduction first gives [B] C6H12, and finally [C] C6H14. [A] on ozonolysis followed by hydrolysis gives two aldehydes [D] C2H4O, and [E] C2H2O. Oxidation of [B] with acidified KMnO4 gives acid [F] C4H8O2. Determine the structures of the compounds [A] to [F] with proper reasoning.
View SolutionA substance C_(4)H_(10)O yields on oxidation a compound C_(4)H_(8)O which gives an oxime and a positive iodoform test. The original substance on treatment with conc. H_(2)SO_(4) gives C_(4)H_(8) . The structure of the compound is
View SolutionCompound C_(8)H_(9)Cl (A) on treatment with KICN followedd by hydrolysis gives C_(9) H_(10) O_(2) (B) . Ammonium salt of (B) on dry disillation yiels (C) , wyhich reacts with alkene solution of bromnine to give C_(8) H_(141) N (D) . Another compound C_(8) H_(10) O (E) is obtained by the actions by the action of nitrous acid of (D) or by the action of aqueosu potacsh on (A), (E) on oxidation gives the inner anthydide C_(8) H_(6)O_(4) (F) which gaves the inner anthdride C_(8) H_(4) O_(3) (G) on heating Identify the various compounds (A) to (G) .
View SolutionAn organic compound (A) of molecular formula C5H8 when treated with Na in liquid ammonia followed by reaction with s-Propyl iodide yeidls (B) C8H12 (A) gives a ketone C5H10O (e) when treated with dil H2SO4 and HgSO4 (B) on oxidation with alkaline KMnO4 gives two isomeric acids (D) and (E) C4H8O2. Give structures of compounds (A) to (E) with proper reasoning .
View SolutionA substance C4H10O yield on oxidation C4H5O which gives oxime and positive iodoform test.
The original substance on treatment with conc. H2SO4 gives C4H8. The structure of compound is
View SolutionMolecular formula C_(4)H_(8)O oxidation gives acetic acid . The structure of C_(4)H_(8)O_(2) will be
View Solution

