Indicate the wrong statement :-
Generally a sigma bond has free rotation along the axis
p-orbitals always have only sideway overlapping
s-orbital never form π-bond
There can not be more than one sigma bond between two atoms
The correct Answer is:B
Topper's Solved these Questions
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
Explore 113 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:p-BLOCK ELEMENT
Explore 80 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
Explore 5 Videos
- HYDROGENBook:ALLENChapter:HYDROGENExercise:EXERCISE-5
Explore 18 Videos
- Ionic EquilibriumBook:ALLENChapter:Ionic EquilibriumExercise:All Questions
Explore 26 Videos
Similar Questions
The oxidation state of Mo in its oxido-complex species [Mo2O4(C2H4)(H2O)2]2− is
View SolutionThe main factor responsible for weak acidic nature of B-F bonds in BF3 is :-
View Solution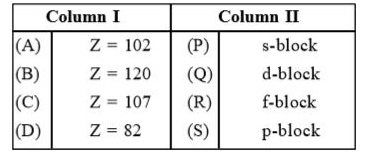
View SolutionAssertion : Cu react with HCl & form H_(2) gas
Reason : Cu above then hydrogen in electro chemical series
View SolutionThe percentage degree of hydrolysis of a salt of weak acid (HA) and weak base (BOH) in its 0.1 M solution is found to be 10%. If the molarity of the solution is 0.05 M, the percentage hydrolysis of the salt should be :
View SolutionThe wrong statement is
View SolutionWhich of the following order is wrong with respect to property indicated?
View SolutionFor the indicator diagram given below, select wrong statement?
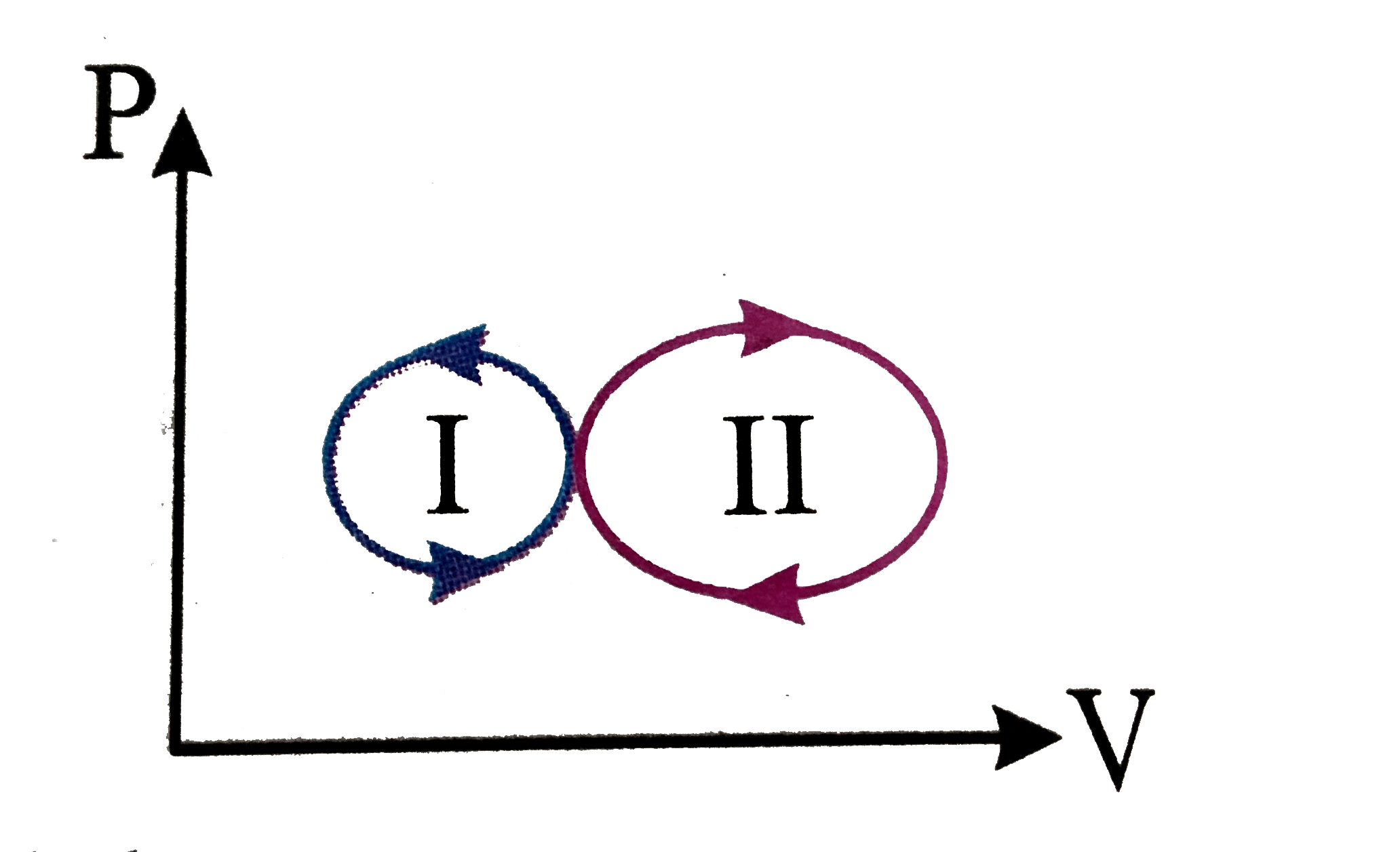
View SolutionIndicate the wrong statement on the basis of the periodic table.
View SolutionIndicate the 'wrong' statement from the following :
Modulation is used to
View SolutionSelect the wrong odd man out from the following statements indicating the properties of an elastic collision.
View SolutionSelect the wrong odd man out from the following statements indicating the properties of an elastic collision.
View Solutionगलत कथन है
View SolutionIndicate the wrong statement on the basis of the periodic table.
View SolutionWhich of the following is incorrect with respect to the property indicated ?
View SolutionWhich of the following orders is wrong with respect to property indicated :
View Solution

