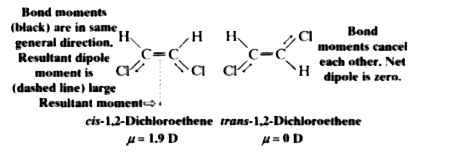
Explain why cis-1.2-dichloroethene (Table 2.1) has a large dipole moment whereas trans-1.2-dichloro. ethene has a dipole moment equal to zero.
लिखित उत्तर
If we examine the net dipole moments (shown in dashed line) for the bond moments (black), we see that in trans-1,2-dichloroethene the bond moments cancel each other, whereas in cis-1.2-dichloroethene they augment each other.
|
टॉपर्स ने हल किए ये सवाल
- FAMILIES OF CARBON COMPOUNDS: FUNCTIONAL GROUPS AND INTERMOLECULAR FORCES BOOK - MS CHOUHANCHAPTER - FAMILIES OF CARBON COMPOUNDS: FUNCTIONAL GROUPS AND INTERMOLECULAR FORCES EXERCISE - ADDITIONAL OBJECTIVE QUESTIONS ( Single Correct Choice Type )
16 Videos
- FAMILIES OF CARBON COMPOUNDS: FUNCTIONAL GROUPS AND INTERMOLECULAR FORCES BOOK - MS CHOUHANCHAPTER - FAMILIES OF CARBON COMPOUNDS: FUNCTIONAL GROUPS AND INTERMOLECULAR FORCES EXERCISE - ADDITIONAL OBJECTIVE QUESTIONS ( Integer Type )
4 Videos
- CONJUGATED UNSATURATED SYSTEMSBOOK - MS CHOUHANCHAPTER - CONJUGATED UNSATURATED SYSTEMSEXERCISE - Additional Objective Questions (Multiple Correct Choice Type)
4 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - MS CHOUHANCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Exercise
279 Videos
समान प्रश्न
ज्ञान जांच
Which has zero dipole moment
ACis-2-buteneBTrans-2-buteneC1-buteneD2-methyl-1-propeneWhich of the following compound has zero dipole moment?
ACH2Cl2BCH4CNH3DPH3Dipole moment of cis-2, 3-dichloro-2 butne is ….. Than the dipole moment of cis1, 2-dichloroethene.
AmoreBlessCneither more nor lessDNone of the above
Which of the following has zero dipole moment?
View SolutionWhich of the following has zero dipole moment?
View SolutionWhich of the following molecules has a dipole moment of zero?
View SolutionH2O has net dipole moment while BeF2 has zero dipole moment because
View SolutionThe molecule which has zero dipole moment is
View Solution

