Fill in the blanks :
________ is added to very dilute nitric acid to produce H2.
To fill in the blank for the question "________ is added to very dilute nitric acid to produce H2", we will go through the following steps:
Step 1: Identify the Reaction
We need to determine which metal reacts with very dilute nitric acid to produce hydrogen gas (H₂).
Hint: Think about common metals that can displace hydrogen from acids.
Step 2: Select the Metal
Magnesium (Mg) is a metal that can react with dilute acids.
Hint: Consider metals that are more reactive than hydrogen.
Step 3: Write the Chemical Equation
When magnesium reacts with very dilute nitric acid (HNO₃), it produces magnesium nitrate (Mg(NO₃)₂) and hydrogen gas (H₂). The balanced chemical equation for this reaction is:
\(
\text{Mg} + 2\text{HNO}_3 \rightarrow \text{Mg(NO}_3\text{)}_2 + \text{H}_2
\)
Hint: Remember that balancing chemical equations involves making sure the number of atoms for each element is the same on both sides.
Step 4: Conclusion
Thus, the answer to the fill-in-the-blank question is "Magnesium".
Final Answer: Magnesium is added to very dilute nitric acid to produce H₂.
Summary of Steps:
1. Identify the reaction between a metal and dilute nitric acid.
2. Select magnesium as the metal.
3. Write the balanced chemical equation for the reaction.
4. Conclude that magnesium is the answer.
We need to determine which metal reacts with very dilute nitric acid to produce hydrogen gas (H₂).
Magnesium (Mg) is a metal that can react with dilute acids.
When magnesium reacts with very dilute nitric acid (HNO₃), it produces magnesium nitrate (Mg(NO₃)₂) and hydrogen gas (H₂). The balanced chemical equation for this reaction is:
\(
\text{Mg} + 2\text{HNO}_3 \rightarrow \text{Mg(NO}_3\text{)}_2 + \text{H}_2
\)
Thus, the answer to the fill-in-the-blank question is "Magnesium".
1. Identify the reaction between a metal and dilute nitric acid.
2. Select magnesium as the metal.
3. Write the balanced chemical equation for the reaction.
4. Conclude that magnesium is the answer.
Topper's Solved these Questions
Similar Questions
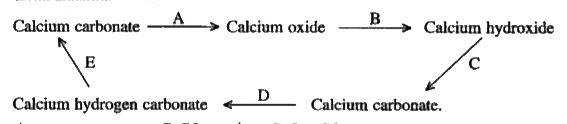
Write equation for each conversion A to E.
View SolutionName a gas which reduces hot copper (II) oxide to copper.
View SolutionGive reasons :
Sodium is kept in an inert solvent.
View SolutionThe metals which produce hydrogen only with very dilute nitric acid are.
View SolutionWhen zinc reacts with very dilute nitric acid it produces
View SolutionIron reacts with very dilute nitric acid to produce
View SolutionTin reacts with dilute nitric acid and produces:
View SolutionThe treatment of zinc with very dilute nitric acid produces:
View SolutionOn reaction with Mg, very dilute nitric acid produces
View SolutionThe metals which produce hydrogen only with very dilute nitric acid are:
View Solutioncopper and The gas produced by the action of dilute nitric acid is
View SolutionThe metals which produce hydrogen only with very dilute nitric acid are
View Solutionजिंक की ठण्डे एवं अति तनु नाइट्रिक अम्ल के साथ क्रिया पर बनता है
View SolutionWhen a zinc reacts with very dilute nitric acid it produces ............
View SolutionOn reaction with Mg, very dilute nitric acid produces
View SolutionWhen Zn reacts with very dilute nitric acid it produces:
View Solution