Write your observations when dilute sulphuric acid is added to the following:
A metal carbonate (sodium carbonate).
Step-by-Step Text Solution:
1. Identify the Reactants:
- The reactants in this reaction are sodium carbonate (Na2CO3) and dilute sulfuric acid (H2SO4).
2. Write the Chemical Reaction:
- When sodium carbonate reacts with dilute sulfuric acid, the reaction can be represented as:
Na2CO3+H2SO4→Na2SO4+H2O+CO2
3. Observe the Reaction:
- Upon adding dilute sulfuric acid to sodium carbonate, you will observe the following:
- Brisk Effervescence: This is due to the release of carbon dioxide (CO2) gas. The effervescence will be noticeable as bubbles form rapidly.
- Formation of a Salt: Sodium sulfate (Na2SO4) is formed as a product of the reaction.
- Water Formation: Water (H2O) is also produced in the reaction.
4. Test for Carbon Dioxide:
- The carbon dioxide gas produced can be tested by passing it through limewater (a solution of calcium hydroxide). If CO2 is present, the limewater will turn milky due to the formation of calcium carbonate (CaCO3).
5. Conclusion of Observations:
- The main observations when dilute sulfuric acid is added to sodium carbonate are:
- The evolution of CO2 gas indicated by brisk effervescence.
- The gas turns limewater milky, confirming the presence of carbon dioxide.
- The reactants in this reaction are sodium carbonate (Na2CO3) and dilute sulfuric acid (H2SO4).
- When sodium carbonate reacts with dilute sulfuric acid, the reaction can be represented as:
Na2CO3+H2SO4→Na2SO4+H2O+CO2
- Upon adding dilute sulfuric acid to sodium carbonate, you will observe the following:
- Brisk Effervescence: This is due to the release of carbon dioxide (CO2) gas. The effervescence will be noticeable as bubbles form rapidly.
- Formation of a Salt: Sodium sulfate (Na2SO4) is formed as a product of the reaction.
- Water Formation: Water (H2O) is also produced in the reaction.
- The carbon dioxide gas produced can be tested by passing it through limewater (a solution of calcium hydroxide). If CO2 is present, the limewater will turn milky due to the formation of calcium carbonate (CaCO3).
- The main observations when dilute sulfuric acid is added to sodium carbonate are:
- The evolution of CO2 gas indicated by brisk effervescence.
- The gas turns limewater milky, confirming the presence of carbon dioxide.
Topper's Solved these Questions
Similar Questions
Write equation for each conversion A to E.
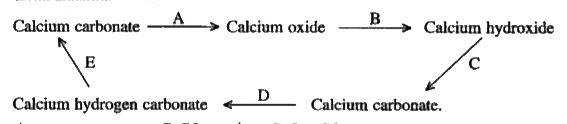
View SolutionName a gas which reduces hot copper (II) oxide to copper.
View SolutionGive reasons :
Sodium is kept in an inert solvent.
View Solutionक्या होता है जब तनु सल्फ्यूरिक अम्ल को ठोस सोडियम कार्बोनेट में मिलाते है ?
View SolutionWrite chemical equations for what happens when a) Sodium metal is added to ethanoic acid. b)Solid sodium carbonate is added to ethanoic acid. c)Ethanoic acid reacts with a dilute solution of sodium hydroxide.
View SolutionState your observation in each of the following cases : (i) When dilute hydrochloric acid is added to sodium carbonate crystals.
View SolutionWrite your observations when dilute sulphuric acid is added to the following: A metal (zinc).
View SolutionWrite your observations when dilute sulphuric acid is added to the following:
A metal sulphide (sodium sulphide).
View SolutionWrite your observations when dilute sulphuric acid is added to the following:
A metal sulphite (potassium sulphite)
View SolutionGive the test of the gas evolved when dilute sulphuric acid is added to the following: Sodium carbonate
View SolutionState your observation : Dilute HCl is added to sodium carbonate crystals.
View SolutionState your observation when:
Dilute hydrochloric acid is added to copper carbonate.
View SolutionExplain the following:
Brisk effervescence is formed on adding dilute sulphuric acid to sodium carbonate.
View SolutionState one appropriate observation for the following:
When dilute hydrochloric acid is added to sodium carbonate crystals.
View SolutionWrite a balanced chemical equation
Action of dilute sulphuric acid on sodium carbonate
View Solution
