Draw an electron dot diagram to show the formation of each of the following compounds :
(i) Methane
(ii) Magnesium Chloride
[H = 1, C = 6, Mg = 12, Cl = 17]
Magnesium chloride
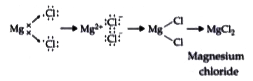
Topper's Solved these Questions
Similar Questions
Arrange the following as per the instruction given in the brackets:
He, Ar, Ne (Increasing order of the number of electron shells)
View SolutionUse the letters only written in the Periodic Table given below to answer the questions that follow :

State the number of valence electrons in atom J.
View SolutionWhat do you understand by a lone pair of electrons ?
View Solutionसोडियम क्लोराइड एवं मेग्नीशियम ऑक्साइड की इलेक्ट्रान-डॉट संरचना (सूत्र) लिखिए । परमाणु क्रमांक-Na=11,Mg=12,Cl=17,O=8.
View Solutionयदि मैग्नीशियम तथा क्लोरीन के परमाणु क्रमांक क्रमशः 12 तथा 17 हैं , तो बताइए कि निम्नलिखित में से प्रत्येक में कितने इलेक्ट्रॉन होंगे -(i) Mg++ (ii)Cl− .
View Solutiona. Write electron dot diagram for chlorine (At No. 17) and Calcium (At No 20) show the formation of Calcium chloride by transfer of electrons.
View Solution(i) Write the electron-dot structures for sodium (11) oxygen (8), chlorine (17) and magnesium (12) show the formation of Na_(2)O and MgO by the transfer of electrons. (ii) Name the ions present in these compounds.
View SolutionDraw the electron dot diagram of following compounds. [Hint: Atomic No. Na=11,F=9,Mg=12]
View SolutionDraw the electron dot diagram of the formation of C Cl_4 , molecule (Hint at . no. C =6 CI =17)
View Solutionनिम्न परमाणुओं के इलेक्ट्रोनिक संरचना आरेख बनाईये ।
Na=11 Cl=17 Mg=12
View SolutionExplain with the help of (i) an ionic equation (ii) electron dot structural diagram (iii) atomic or orbital structural diagram for the formation of the following:
(a) Sodium chloride (b) Calcium oxide (c) Magnesium chloride.
View SolutionDraw an electron dot diagram to show the formation of each of the following compounds :
(i) Methane
(ii) Magnesium Chloride
[H = 1, C = 6, Mg = 12, Cl = 17]
View SolutionDraw an electron dot diagram to show the formation of ammonium ion [N = 7, H = 1]
View SolutionExplain with the help of [i] an ionic equation [ii] electron dot structural diagram - the formation of the following [a] Sodium chloride [b] Calcium oxide [c] Magnesium chloride. [at.nos. Na = 11, Cl = 17, Ca = 20, O = 8, Mg = 12].
View SolutionBy drawing an electron dot diagram show the formation of Ammonium Ion [Atomic No. : N = 7 and H = 1]
View SolutionDraw the electron dot diagram for the compounds given below. Represent the electrons by (.) and (x) in the diagram : [Atomic No. Ca = 20, O = 8, Cl = 17, H = 1] Calcium oxide
View Solution