The following data were obtained for thermal decomposition of N2O5(g) at constant volume.
2N2O5(g)→2N2O4(g)+O2(g)
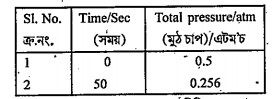
Calculate the rate constant.Solution in Assamese
Step by step video solution for The following data were obtained for thermal decomposition of N_2O_5(g) at constant volume. 2N_2O_5(g) rarr 2N_2O_4(g)+O_2(g) Calculate the rate constant. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Mention the hydrolysis products of sucrose.
View SolutionWhat is the composition of tincture of iodine?
View SolutionThe following data were obtained during the first order decomposition of SO_(2)Cl_(2) at a constant volume: SO_(2)Cl_(2)(g) to SO_(2)(g) + Cl_(2)(g) Calculate the rate constant.
View Solution2N2O5(g)→4NO2(g)+O2(g) what is the ratio of the rate of decomposition of N2O5 to rate of formation of O2 ?
View SolutionThe following data were obtained during the first order thermal decomposition of N2O5 at constant volume :
2N2O5→2N2O4+O2

Calculate rate constant.
View SolutionIn the process 2N_2O_5(g) to 4NO_2(g) + O_2(g) , at t = 10 rate of reaction w.r.t N_2O_5, NO_2 & O_2 respectively are
View SolutionThe value of equilibrium constant for the reaction [N2O5(g)⇒2NO2(g)+12O2(g)] is 0.5. The equilibruim constant for the reaction [4NO2(g)+O2(g)⇒2N2O5(g)] is
View Solutionस्थिर आयतन पर N2O5(g) के प्रथम कोटि के तापीय वियोजन पर निम्न आँकड़े प्राप्त हुए।
2N2O5(g)→2N2O4(g)+O2(g)
क्रम संख्या समय (सेकण्ड) कुल दाब (वायु)(i)00.5(ii)1000.512
वेग स्थिरांक की गणना कीजिए।
View SolutionFor the reaction 2N_2O_5(g) rarr 4NO_2(g)+O_2(g) the following results have been obtained. Calculate rate constant of the reaction. 2N_2O_5(g)rarr4NO_2(g)+O_2(g)
View SolutionConsider that the first order decomposition reaction of N_2O_5 written as 2N_2O_5(g) rarr 4NO_2(g) + O_2(g): rate = k[N_2O_5] N_2o_5(g) rarr 2NO_2(g) +1/2 O_2(g): rate = k^'[N_2O_5] Which among the following one is correct?
View SolutionThe value of equilibrium constant for the reaction [N_2O_5 (g) hArr 2NO_2(g) + 1/2 O_2(g)] is 0.5. The equilibruim constant for the reaction [4NO_2(g) + O_2(g) hArr 2N_2O_5(g)] is
View SolutionThe following data were obtained during the first order thermal decomposition of N_2O_5 (g) at constant volume: 2N_2O_5 (g) rarr 2N_2O_4 (g) +O_2(g)
View Solution2N_2O_5(g)rarr4NO_2(g)+O_2(g) what is the ratio of the rate of decomposition of N_2O_5 to rate of formation of O_2 ?
View SolutionThe rate coristant for the reaction 2N2O5(g)→2N2O4(g)+O2(g) is 4.98×10−4s−1. The order of reaction is
View SolutionThe following data were obtained during the first order thermal decomposition of N2O5(g) at constant volume:
2N2O5(g)→2N2O4(g)+O2(g)

View Solution
