The following data were obtained at a certain temperature for the decomposition of ammonia in contact with tungsten:
p (m m Hg) 50 100 200
Relative t1/2 3.64 1.82 0.91
Find the order of the reaction.
We know ,
(t12)1(t12)2=(a2a1)n−1 ...(Eqn.13)
3.641.82=(10050)n−1
2=2n−1
n−1=1
n = 2
Topper's Solved these Questions
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:PROBLEMS
Explore 42 Videos
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:OBJECTIVE PROBLEMS
Explore 51 Videos
- CHEMICAL EQUIVALENCEBook:RC MUKHERJEEChapter:CHEMICAL EQUIVALENCEExercise:PROBLEMS
Explore 24 Videos
- CHEMICAL THERMODYNAMICSBook:RC MUKHERJEEChapter:CHEMICAL THERMODYNAMICSExercise:Objective problems
Explore 52 Videos
Similar Questions
The following data were obtained at a certain temperature for the decomposition of ammonia in contact with tungsten:
p (m m Hg) 50 100 200
Relative t1/2 3.64 1.82 0.91
Find the order of the reaction.
View SolutionIn te decomposition of Ammonia it was found that at 50 torr pressure t(1/2) was 3.64 hour while at 100 torr t(1/2) was 1.82 hours. Then order of reaction would be:
View Solution
Knowledge Check
The following data were obtained at a certain temperature for the decomposition of NH3 in contact with catalyst.
P(mn)2550t1/21.820.91
The order of reaction isA2B3C1D4At certain temperature, the half life period in the thermal decomposition of a gaseous substance as follows :
P (mm Hg) 500 250
t1/2 (in min) 235 950
Find the order of reaction [Given log (23.5)=1.37, log (95)=1.97]A1B2C2.5D3The following data were obtained for the thermal decomposition of nitrous oxide on finely divided gold :
t (minutes)020406080Concentration of N2O(molL−1)0.100.080.060.040.02
The order of reaction is :AzeroB1C2D3
At a certain temperature the half life periods of a reactio at different initial pressure were as follows :
p(kPa)10066.6733.33t(min)150235950
Find out the order or the reaction.
View SolutionHalf lives of decomposition of NH3 on the surface of a catalyst for different initial pressure are given as :
P(torr) 200 300 500
t1/2 10 15 25
The order of the reaction is −
View SolutionFor the decompoistion of HI at 1000K(2HI→H2+I2), following data were obtained:
∣∣ ∣ ∣ ∣ ∣∣[HI](M)Rate of decomposition of HI(molL−1s−1)0.12.75×10−80.211×10−80.324.75×10−8∣∣ ∣ ∣ ∣ ∣∣
The order of reaction is
View SolutionThe following data were obtained during the first order thermal
decomposition of SO2Cl2 at a constant volume SO2Cl2(g)→SO2(g)+Cl2(g)
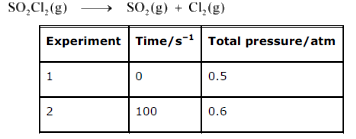
Calculate the rate of the reaction when total pressure is 0.65 atm
View SolutionFor the following data answer the questions :
Reaction : A+B → P
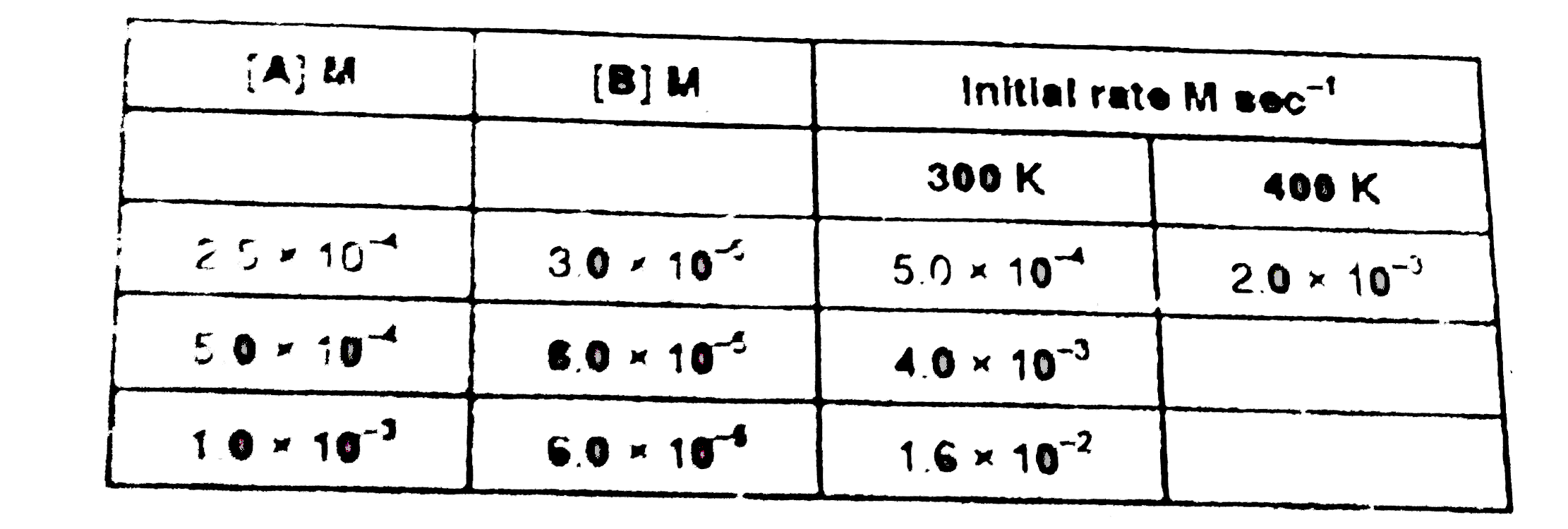
The value of rate constant at 300 K is (M−2sec−1):
View Solution
