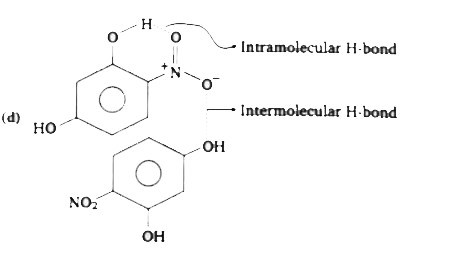
The type of molecular forces of attraction present in the following compounds is :
Intermolecular H-bonding
Intramolecular H-bonding
van der waal's force
All of these
The correct Answer is:D
Topper's Solved these Questions
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 3
Explore 72 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (ADVANCED)Exercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 77 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (ADVANCED)Exercise:SUBJECTIVE PROBLEMS
Explore 48 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (BASIC)Exercise:SUBJECTIVE PROBLEMS
Explore 49 Videos
Similar Questions
Colour of compounds occurs due to phenomenon of polarisation, charge transfer, d-d transition and H.O.M.O.L.U.M.O transition. Most of the transition metal transition metal complex compound are coloured either due to d-d electrons transition or charge transfer and ionic compounds are coloured due to polarisation of anion. Q. Which of the following is correct about KFeII[FeIII(CN6)]andKFeIII[FeII(CN6)] complex compounds?
View SolutionWhich is correct statement ?
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionConsider following compounds A to E :
(A) XeFn (B)XeF+(n+1) (C)XeF−(n+1) (D)XeF(n+2)
(E ) XeF2−(n+4),
If value of n is 4, then calculate value of p÷q here, 'p' is total number of bond pair and 'q' is total number of lone pair on central atoms of compounds (A) to (E ).
View SolutionThe attractive forces between molecules of a molecular crystal are
View Solutionअन्तरा-अणुक आकर्षक बल नगण्य होता है
View Solutionअन्तरा-अणुक आकर्षण बल प्रबल होते हैं
View Solutionआण्विक क्रिस्टल में अणुओं के मध्य किस प्रकार का आकर्षण बल होता है ?
View SolutionWhich of the following order of molecular force of attraction among given species is incorect ?
View SolutionThe type of molecular forces of attraction present in the following compounds is :
View SolutionThe intermolecular forces of attraction (i.e., H-bonding and van der waal's forces) exist among polar and non-polar species which effect melting point, boiling point, solubility and viscosity of covalent compounds : Q.The type of molecular force of attraction present in the following compounds is :
View SolutionThe types of attractive force between a polar molecular and non - polar molecule are
View SolutionWhich of the following order of molecular force of attraction among given species is incorect ?
View SolutionThe type of molecular forces of attraction present in the following compounds is :
View SolutionThe intermolecular forces of attraction (i.e., H-bonding and van der waal's forces) exist among polar and non-polar species which effect melting point, boiling point, solubility and viscosity of covalent compounds :
Q.The type of molecular force of attraction present in the following compounds is :
View SolutionName the type of attractive forces present in (a) elastomers (b) fibrous polymers.
View Solution