Of the following reduction processes, correct processes are :
Fe2O3+C→Fe
ZnO+C→Zn
Ca3(PO4)2+C→P
PbO+C→Pb
The correct Answer is:A, B, C, D
To determine which of the reduction processes listed in the question are correct, we will analyze each option based on the principles of metallurgy and thermodynamics.
1. Understanding Reduction Processes:
- Reduction is a chemical process where a substance gains electrons, often involving the removal of oxygen or the addition of hydrogen. In metallurgy, reduction is commonly used to extract metals from their ores.
2. Analyzing Each Option:
- Option 1: Iron Oxide + Carbon → Iron
- This reaction is a valid reduction process. Carbon can reduce iron oxide (FeO or Fe2O3) to iron (Fe) at high temperatures. This process is commonly used in blast furnaces.
- Option 2: Zinc Oxide + Carbon → Zinc
- This reaction is also a valid reduction process. Zinc oxide (ZnO) can be reduced to zinc (Zn) using carbon as a reducing agent at high temperatures (around 1673 K). This reaction is spontaneous under these conditions.
- Option 3: Ca3(PO4)2 + Carbon → Phosphorus
- This reaction is not a typical reduction process for phosphorus. Calcium phosphate does not directly yield phosphorus through carbon reduction. Phosphorus is usually obtained from phosphate rock through a different process, often involving high temperatures and silica.
- Option 4: Lead Oxide + Carbon → Lead
- This reaction is a valid reduction process. Lead oxide (PbO) can be reduced to lead (Pb) using carbon as a reducing agent. This is commonly done in metallurgy.
3. Conclusion:
- The correct reduction processes from the options provided are:
- Option 1: Iron Oxide + Carbon → Iron (Correct)
- Option 2: Zinc Oxide + Carbon → Zinc (Correct)
- Option 4: Lead Oxide + Carbon → Lead (Correct)
- Option 3 is incorrect.
Final Answer:
The correct reduction processes are Option 1, Option 2, and Option 4.
- Reduction is a chemical process where a substance gains electrons, often involving the removal of oxygen or the addition of hydrogen. In metallurgy, reduction is commonly used to extract metals from their ores.
- Option 1: Iron Oxide + Carbon → Iron
- This reaction is a valid reduction process. Carbon can reduce iron oxide (FeO or Fe2O3) to iron (Fe) at high temperatures. This process is commonly used in blast furnaces.
- Option 2: Zinc Oxide + Carbon → Zinc
- This reaction is also a valid reduction process. Zinc oxide (ZnO) can be reduced to zinc (Zn) using carbon as a reducing agent at high temperatures (around 1673 K). This reaction is spontaneous under these conditions.
- Option 3: Ca3(PO4)2 + Carbon → Phosphorus
- This reaction is not a typical reduction process for phosphorus. Calcium phosphate does not directly yield phosphorus through carbon reduction. Phosphorus is usually obtained from phosphate rock through a different process, often involving high temperatures and silica.
- Option 4: Lead Oxide + Carbon → Lead
- This reaction is a valid reduction process. Lead oxide (PbO) can be reduced to lead (Pb) using carbon as a reducing agent. This is commonly done in metallurgy.
- The correct reduction processes from the options provided are:
- Option 1: Iron Oxide + Carbon → Iron (Correct)
- Option 2: Zinc Oxide + Carbon → Zinc (Correct)
- Option 4: Lead Oxide + Carbon → Lead (Correct)
- Option 3 is incorrect.
The correct reduction processes are Option 1, Option 2, and Option 4.
Topper's Solved these Questions
- METALLURGYBook:VK JAISWAL ENGLISHChapter:METALLURGYExercise:MATCH THE COLUMN
Explore 20 Videos
- METALLURGYBook:VK JAISWAL ENGLISHChapter:METALLURGYExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 29 Videos
- METALLURGYBook:VK JAISWAL ENGLISHChapter:METALLURGYExercise:Level 3 (Passive 7)
Explore 8 Videos
- ENVIRONMENTAL CHEMISTRYBook:VK JAISWAL ENGLISHChapter:ENVIRONMENTAL CHEMISTRYExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 14 Videos
- p-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:p-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 35 Videos
Similar Questions
Knowledge Check
Which of the following process is correct for given P-V diagram.
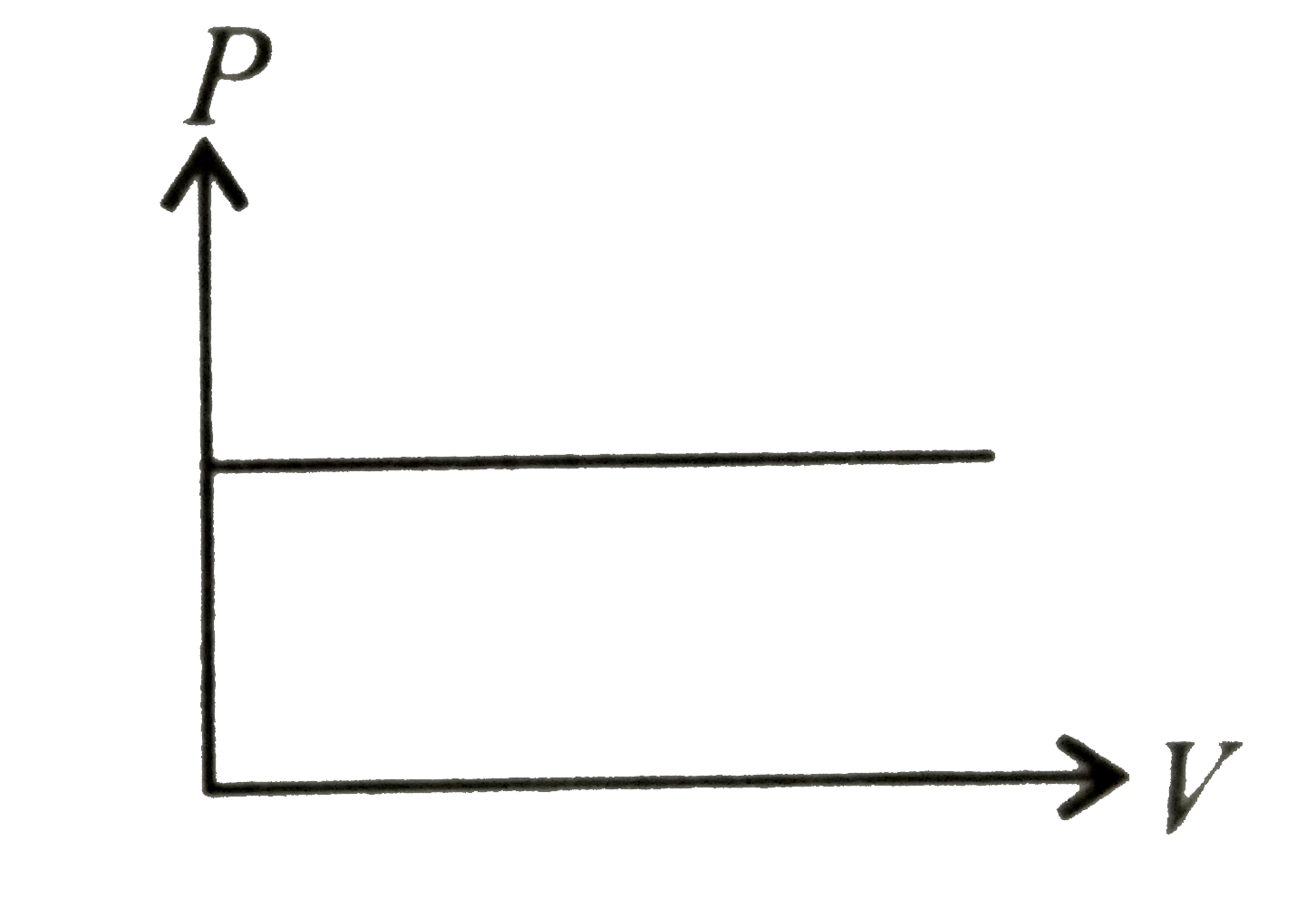 AAdiabatic processBIsothermal processCIsobaric processDIsochoric process
AAdiabatic processBIsothermal processCIsobaric processDIsochoric process
Consider the following reduction processes :
(I) Fe2O3+C→Fe
(II) Al2O3+C→Al
(III) Pbo+C→Pb
(IV) Ca3(PO4)2+C+SiO2→P
The correct processe(s) is/ are
View SolutionOut of the following reduction processes :
(i) Fe2O3+3C→2Fe+3CO
(ii) Cu2O+C→2Cu+CO
(iii) PbO+C→Pb+CO
(iv) ZnO+C→Zn+CO
Correct process is//are :
View SolutionAmong the following processes, select the process for which ΔS is positive.
View SolutionIn the following cyclic process is
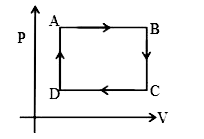
The above process in the P-T coordinates is given as
View SolutionWhich of the following regarding the said processes is (are) correct?
View SolutionIt the following processes, identify the irreversible process :
View SolutionWhich of the following processes involve the roasting process ?
View Solution
